- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记5.1.8 Energy Cycle Calculations
Energy Cycle Calculations
- The energy cycle involving the enthalpy change of solution (ΔHsolꝋ ), lattice energy (ΔHlattꝋ), and enthalpy change of hydration (ΔHhydꝋ) can be used to calculate the different enthalpy values
- According to Hess’s law, the enthalpy change of the direct and of the indirect route will be the same, such that:
ΔHhydꝋ = ΔHlattꝋ + ΔHsolꝋ
- This equation can be rearranged depending on which enthalpy value needs to be calculated
- For example, ΔHlattꝋ can be calculated using:
ΔHlattꝋ = ΔHhydꝋ - ΔHsolꝋ
- Remember: the total ΔHhydꝋ is found by adding the ΔHhydꝋ values of both anions and cations together
- Remember: take into account the number of each ion when completing calculations
- For example, MgCl2 has two chloride ions, so when completing calculations this will need to be accounted for
- In this case, you would need to double the value of the hydration enthalpy, since you are hydrating 2 moles of chloride ions instead of 1
Worked example: Calculating the enthalpy change of hydration of chloride
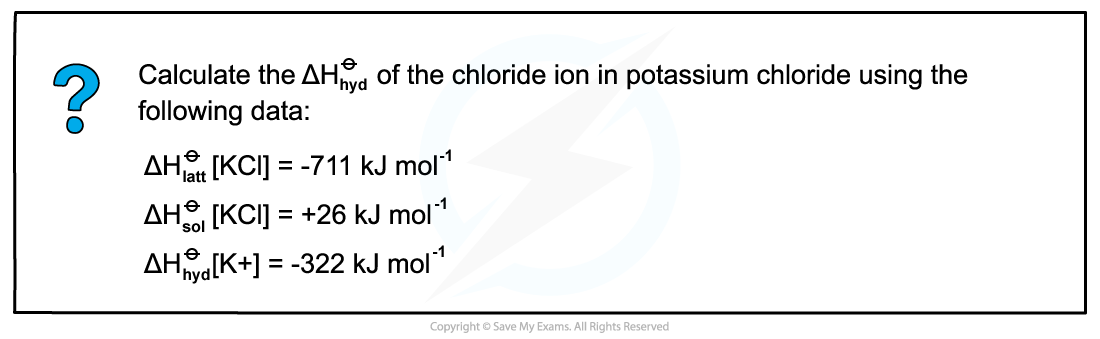
Answer
Step 1: Draw the energy cycle of KCl
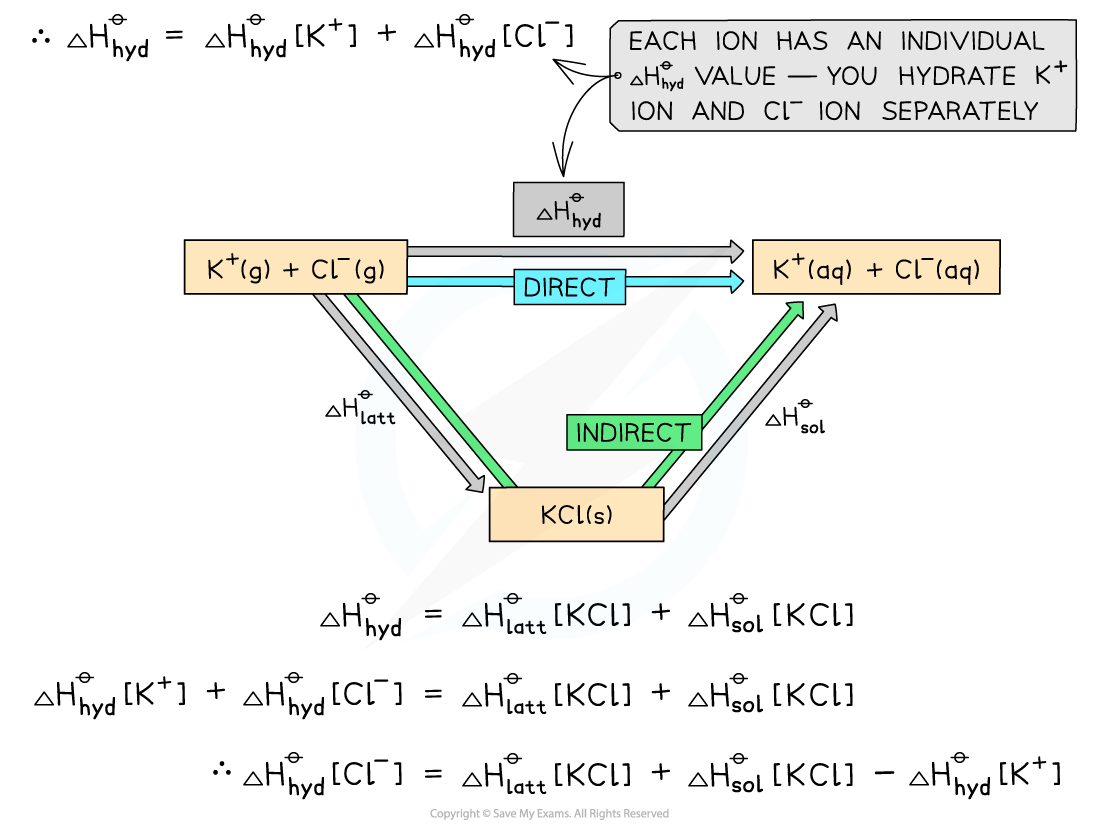
- Step 2: Apply Hess’s law to find ΔHhydꝋ [Cl-]
ΔHhydꝋ = (ΔHlattꝋ[KCl]) + (ΔHsolꝋ[KCl])
(ΔHhydꝋ[K+]) + (ΔHhydꝋ[Cl-]) = (ΔHlattꝋ[KCl]) + (ΔHsolꝋ[KCl])
(ΔHhydꝋ[Cl-]) = (ΔHlattꝋ[KCl]) + (ΔHsolꝋ[KCl]) - (ΔHhydꝋ[K+])
- Step 3: Substitute the values to find ΔHhydꝋ [Cl-]
ΔHhydꝋ [Cl-] = (-711) + (+26) - (-322) = -363 kJ mol-1
Worked example: Calculating the enthalpy change of hydration of magnesium
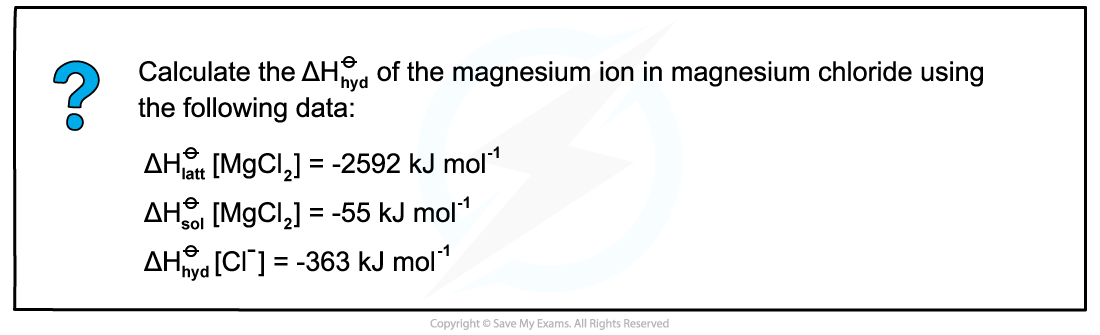
Answer
- Step 1: Draw the energy cycle of MgCl2
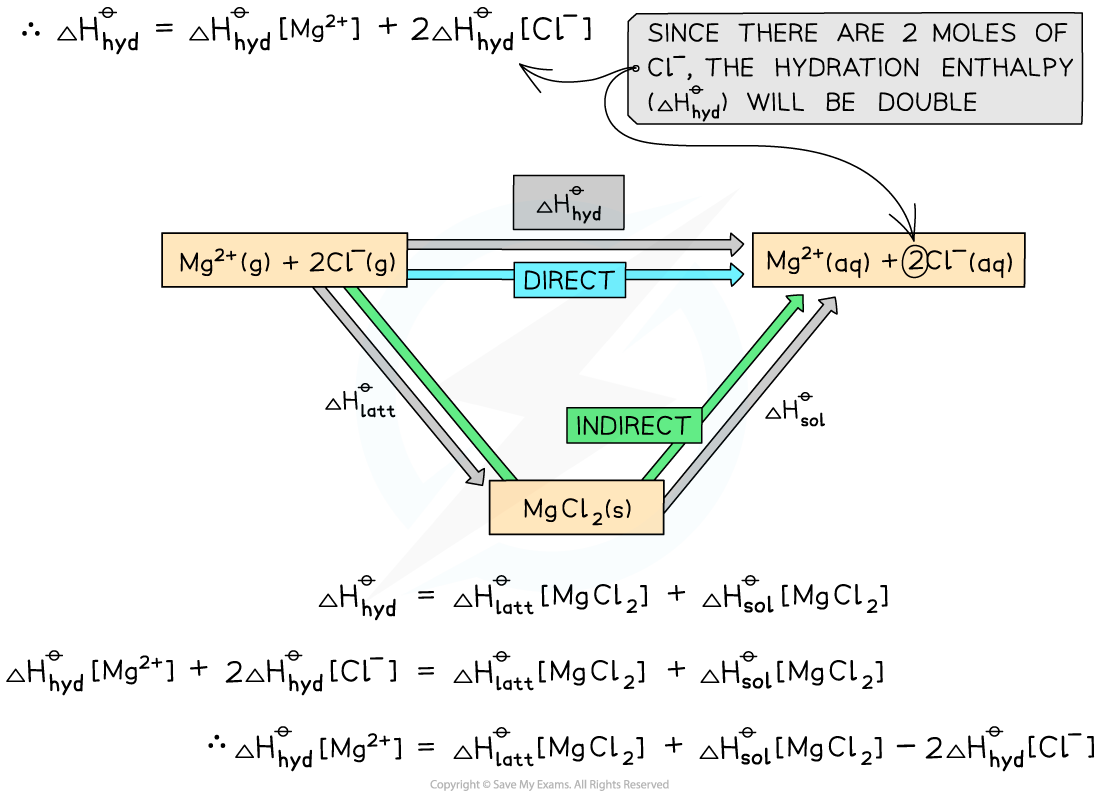
- Step 2: Apply Hess’s law to find ΔHhydꝋ[Mg2+]
ΔHhydꝋ = (ΔHlattꝋ[MgCl2]) + (ΔHsolꝋ [MgCl2])
(ΔHhydꝋ[Mg2+]) + (2ΔHhydꝋ [Cl-]) = (ΔHlattꝋ [MgCl2]) + (ΔHsolꝋ [MgCl2])
(ΔHhydꝋ[Mg2+]) = (ΔHlattꝋ[MgCl2]) + (ΔHsolꝋ[MgCl2]) - (2ΔHhydꝋ[Cl-])
- Step 3: Substitute the values to find ΔHhydꝋ [Mg2+]
ΔHhydꝋ[Mg2+] = (-2592) + (-55) - (2 x -363) = -1921 kJ mol-1
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









