- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记5.1.3 Constructing Born-Haber Cycles
Constructing Born-Haber Cycles
- To calculate the lattice energy (ΔHlattꝋ) of an ionic compound, a Born-Haber cycle is used, which is a special type of energy cycle
- To find the ΔHlattꝋ of an ionic compound, the following values must be known:
- Enthalpy change of formation (ΔHfꝋ)
- The various enthalpy changes involved when going from elements in their standard states to their gaseous ions (the sum of all of these can be referred to as ΔH1ꝋ)
- The various enthalpy changes involved when going from elements in their standard states to their gaseous ions include:
- Enthalpy change of atomisation of each element
- First ionisation energy of the metal
- Successive ionisation energies of the metal if applicable
- First electron affinity of the non-metal
- Successive electron affinities of the non-metal if applicable
- The order that these are written in the Born-Haber cycle is important - they must be done in the order written above
- First ionisation energy cannot take place before atomisation, because first ionisation energy is the process of turning gaseous atoms into gaseous ions
- Electron affinity cannot take place before first ionisation energy, since the electrons must be lost from the metal first during ionisation, to be present in order for the non-metal to gain them
- Hess’s law states that ‘the enthalpy change in a chemical reaction is the same regardless of the route taken, as long as the final and initial conditions and states of reactants and products are the same for each route’
- Although a Born-Haber cycle is usually constructed and used to calculate the lattice energy of an ionic compound, the cycle could be used to calculate any stage in the cycle, since an energy change is the same regardless of the route taken
- For example, the energy cycle shows that there are two routes to go from the elements in their standard states to the ionic compound:
- Each route begins at the same stage of the cycle and ends at the same stage of the cycle
- According to Hess’s law, the enthalpy change for both routes is the same, so the cycle can be used to calculate an unknown value within the cycle
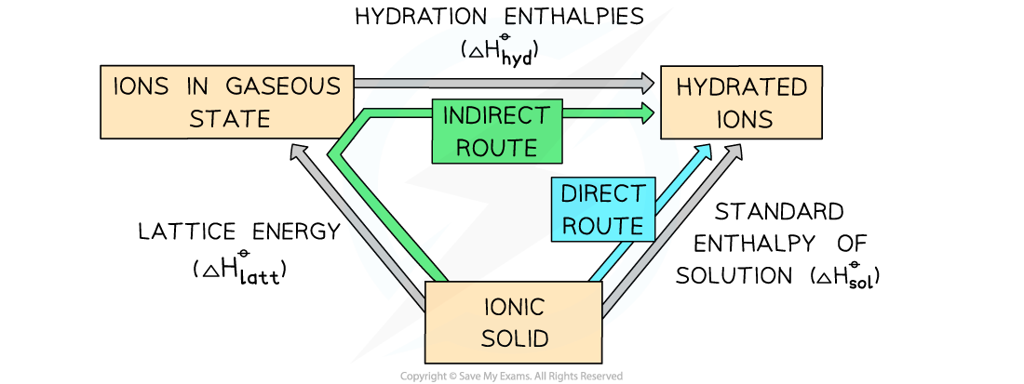
The green arrow shows the indirect route, which is equal in energy to the direct route of the element in the standard states changing into the ionic compound (blue arrow)
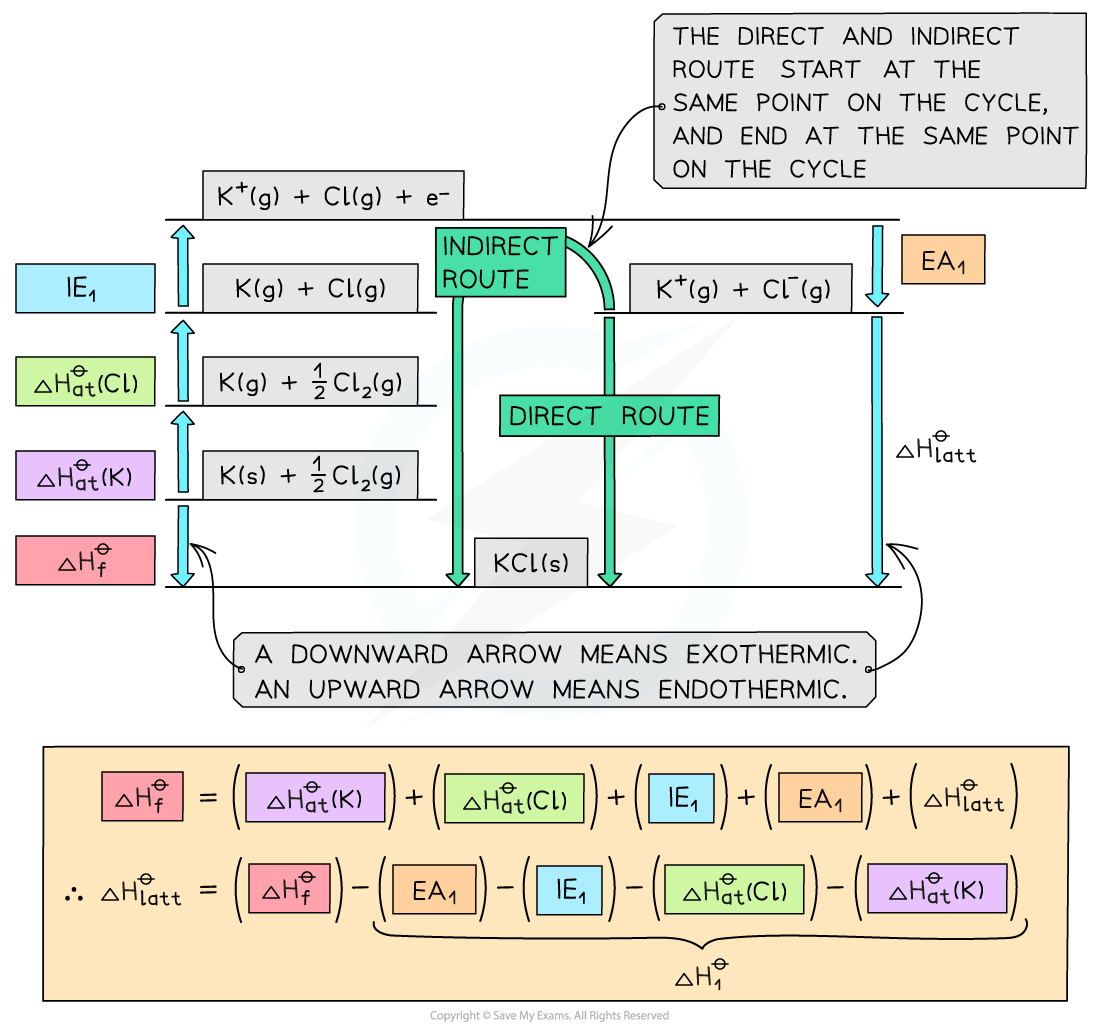
Worked example: Constructing a Born-Haber cycle for KCl

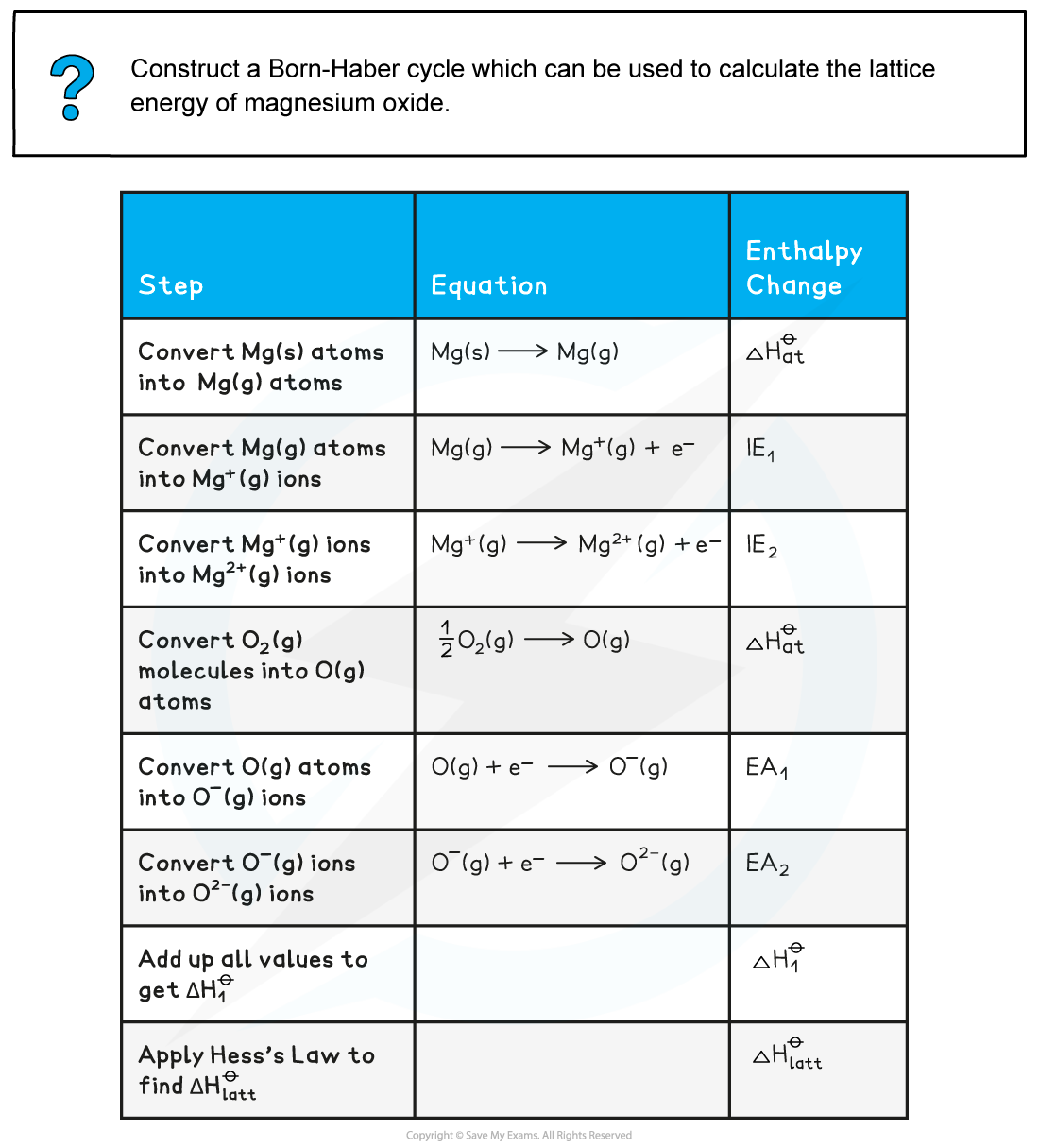
Worked example: Constructing a Born-Haber cycle for MgO

Exam Tip
When constructing Born-Haber cycles, be sure to use ionisation energies (IE) for metals (as metals lose electrons) and electron affinities (EA)for non-metals (as non-metals gain electrons).Remember that the order that the steps go in is important!
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









