- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记5.1.2 Electron Affinity & Trends of Group 16 & 17 Elements
Electron Affinity
Electron affinity
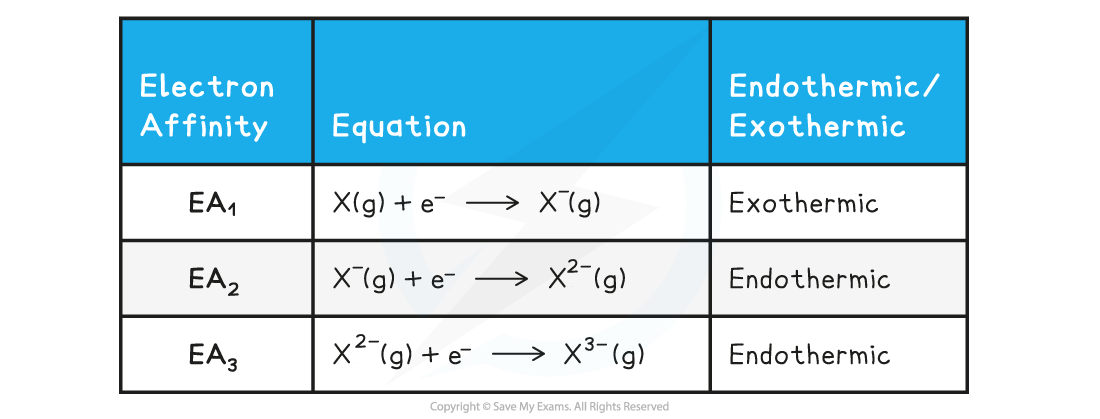
- The first electron affinity (EA1) is the enthalpy change when 1 mole of electrons is added to 1 mole of gaseous atoms, to form 1 mole of gaseous ions each with a single negative charge under standard conditions
X(g) + e- → X-(g)
- EA1 is usually exothermic, as energy is released
- Since this is generally an exothermic process, then the value for EA1 will usually be a negative number
- An element can also accept more than one electron, in which case successive electron affinities are used
- For example, the second electron affinity (EA2) and third electron affinity (EA3) of an element represent the formation of 1 mole of gaseous ions with 2- and 3- charges respectively
- The second and third electron affinities are endothermic, as energy is absorbed
- This is because the incoming electron is added to an already negative ion
- Energy is required to overcome the repulsive forces between the incoming electron and negative ion
- Since these are endothermic processes, the values will be positive
Second & third electron affinity table
Factors affecting electron affinity
- The value of the electron affinity depends on how strongly the incoming electron is attracted to the nucleus
- The greater the attractive forces between the electron and nucleus, the more energy is released and therefore the more exothermic (more negative) the EA1 value will be
- The factors affecting the electron affinity of an element are the same as those that affect the ionisation energy (the formation of positive ions via the loss of electrons)
- These are:
- Nuclear charge: the greater the nuclear charge, the stronger the attractive forces between an incoming electron and the nucleus
- Distance: the greater the distance between the nucleus and the outermost shell/orbital where the electron is added, the weaker the force of attraction
- Shielding: the greater the number of shells, the greater the shielding effect and the weaker the force of attraction
Trends in electron affinity of Group 16 & Group 17 elements
- Electron affinities of non-metals become more exothermic across a period, with a maximum at Group 17
- There is generally a downwards trend in the size of the electron affinities of the elements in Group 16 and 17
- The electron affinities generally become less exothermic for each successive element going down both Groups, apart from the first member of each Group (oxygen and fluorine respectively)
Electron affinity table
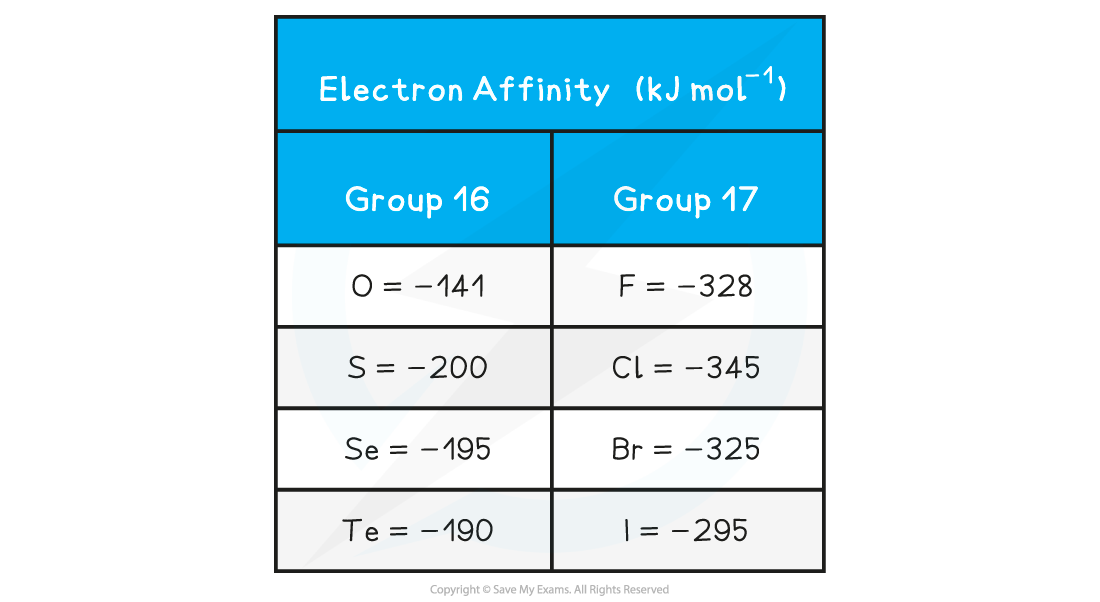
- An atom of chlorine has a greater nuclear charge than an atom of sulfur
- Chlorine will therefore have a greater attractive force between its nucleus and its outer electrons
- More energy is released upon adding an electron to chlorine, so the EA1of Cl is more exothermic than for S
- Going down Group 16 and 17:
- The outermost electrons are held less tightly to the nucleus as they are further away
- The number of electron shells increases causing an increased shielding of the outermost electrons
- It gets more difficult to add an electron to the outer shell
- Less energy is released upon adding an electron to the outer shell
- So generally, the EA1becomes less exothermic
- Fluorine is an exception and has a lower EA1 than chlorine
- Fluorine has a very small atomic radius
- This means that the electron density of fluorine is high
- There is more repulsion between the incoming electron and the electrons that are already present in fluorine
- These repulsive forces reduce the attractive forces between the incoming electron and nucleus
- As a result, the EA1 of fluorine is less exothermic than expected
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








