- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记4.1.6 M + 2 Peak
Detecting Bromine & Chlorine Atoms Using M+2 Peak
- The presence of bromine or chlorine atoms in a compound gives rise to a [M+2] and possibly [M+4] peak
Chlorine
- Chlorine exists as two isotopes, 35Cl and 37Cl
- A compound containing one chlorine atom will therefore have two molecular ion peaks due to the two different isotopes it can contain
- 35Cl = M+ peak
- 37Cl = [M+2] peak
- The ratio of the peak heights is 3:1 (as the relative abundance of 35Cl is 3x greater than that of 37Cl)
- A compound containing two chlorine atoms will have three molecular ion peaks due to the different combinations of chlorine isotopes they can contain
- 35Cl + 35Cl = M+ peak
- 35Cl + 37Cl = [M+2] peak
- 37Cl + 37Cl = [M+4] peak
- The ratio of the peak heights is 9:6:1
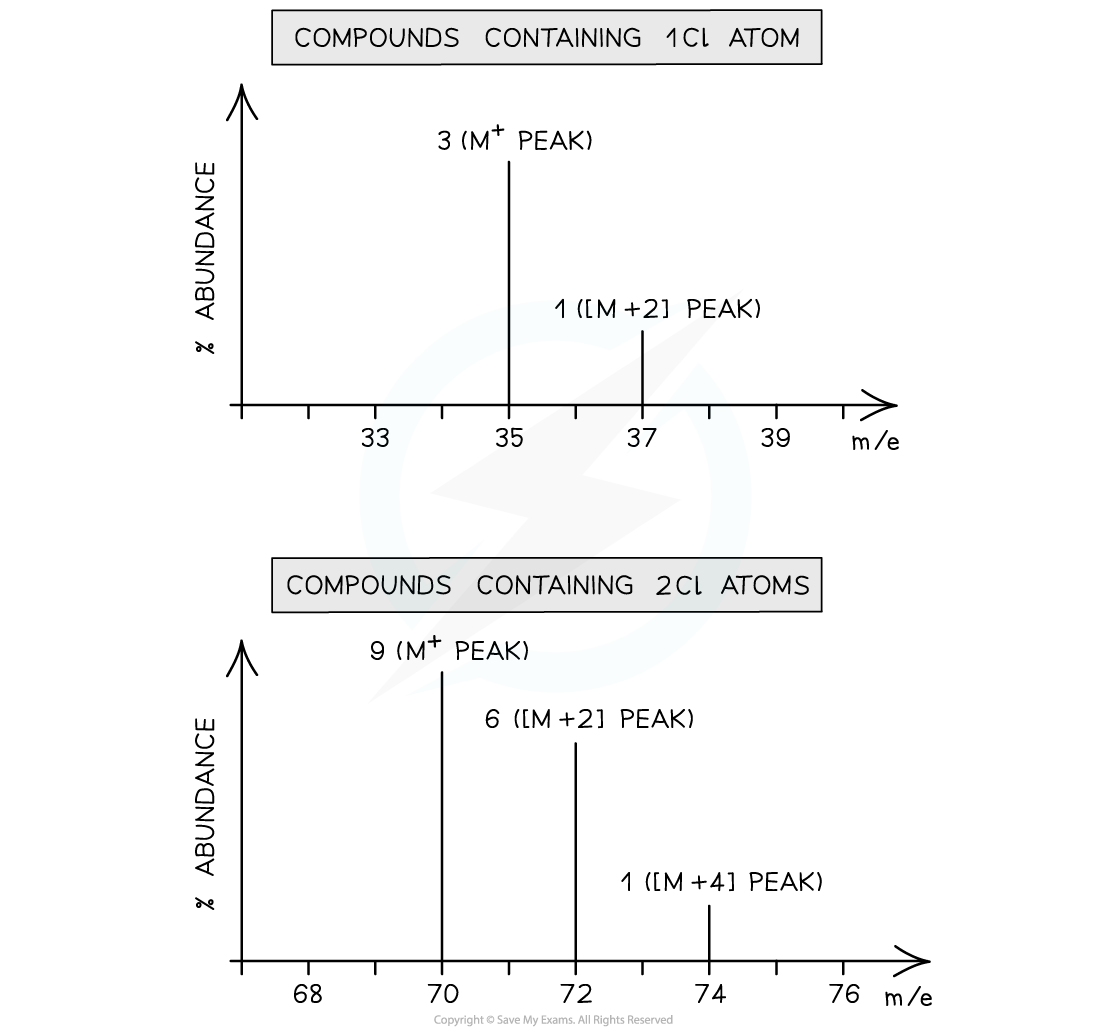
Mass spectrum of compounds containing one chlorine atom (1) and two chlorine atoms (2)
Bromine
- Bromine too exists as two isotopes, 79Br and 81Br
- A compound containing one bromine atom will have two molecular ion peaks
- 79Br = M+ peak
- 81Br = [M+2] peak
- The ratio of the peak heights is 1:1 (they are of similar heights as their relative abundance is the same!)
- A compound containing two bromine atoms will have three molecular ion peaks
- 79Br + 79Br= M+ peak
- 79Br+ 81Br = [M+2] peak
- 81Br + 81Br= [M+4] peak
- The ratio of the peak heights is 1:2:1
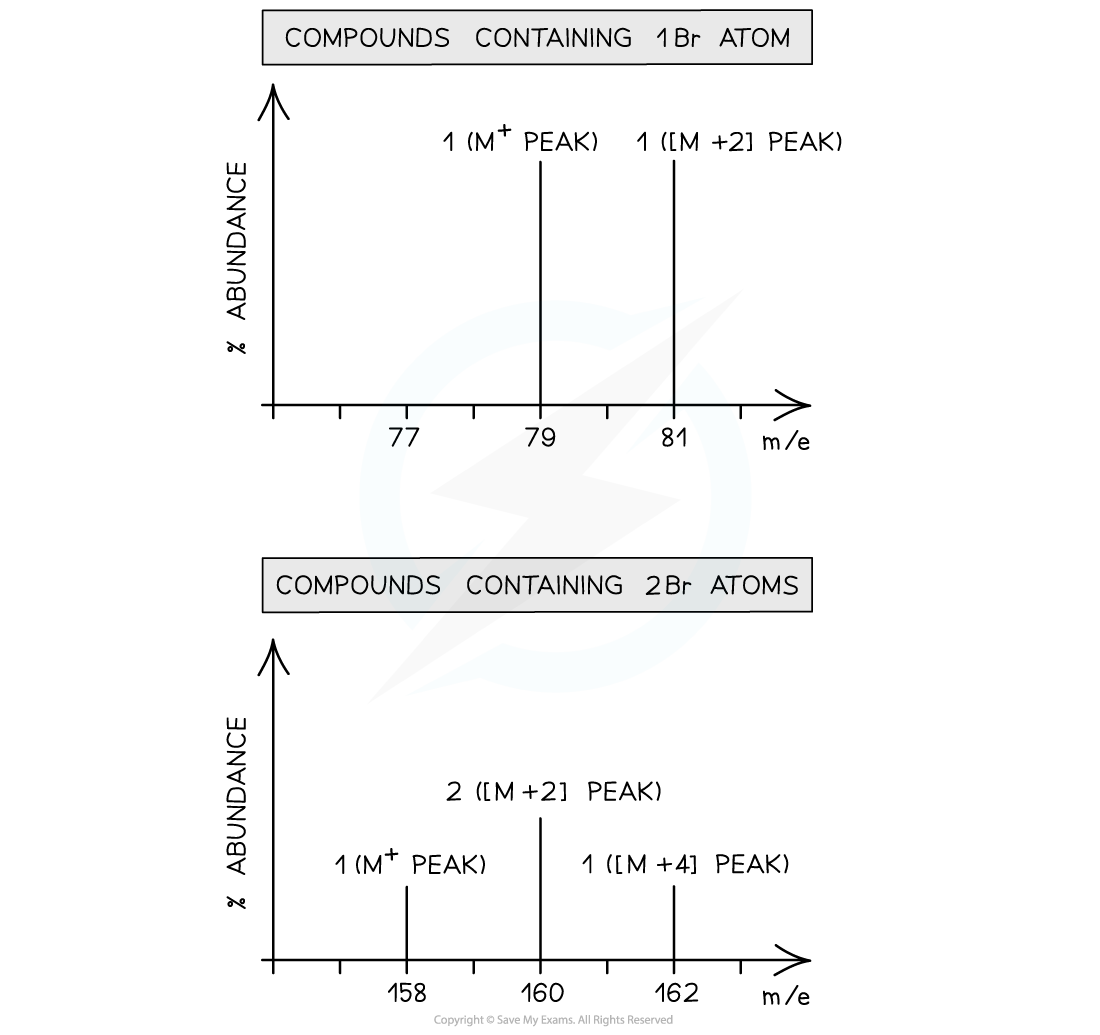
Mass spectrum of compounds containing one bromine atom
Worked example: Analysing bromine spectra

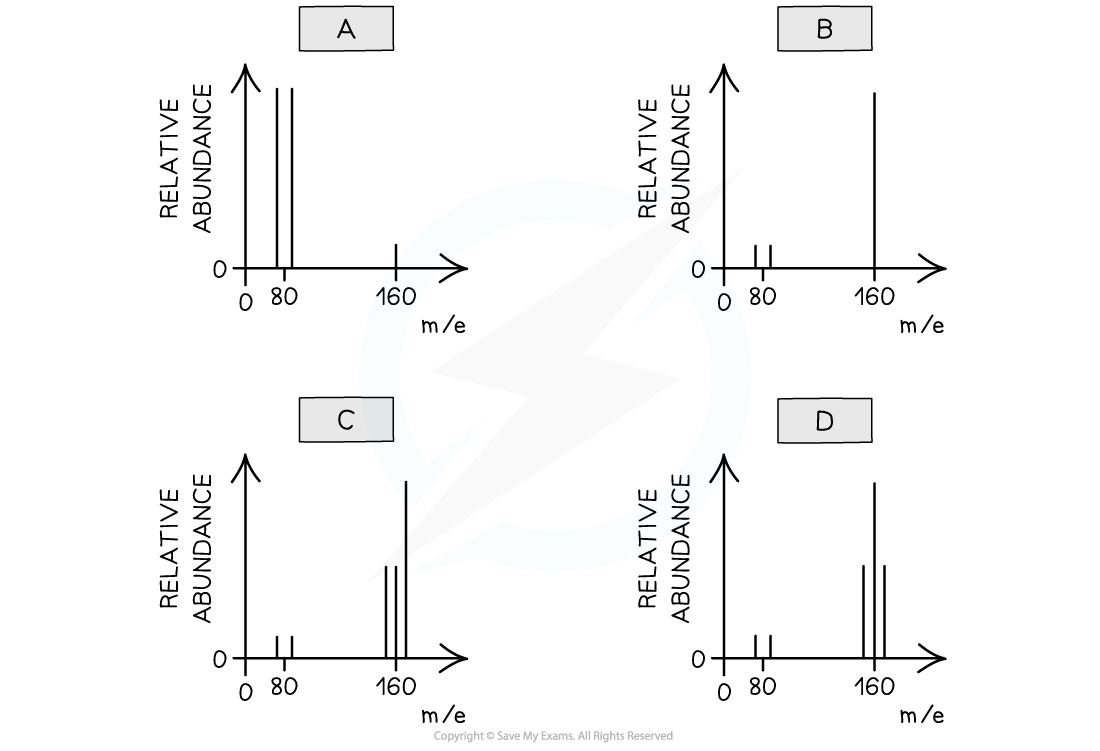
Answer
The correct answer is D
Bromine is a diatomic molecule there will be 5 peaks on the mass spectrum of bromine
Bromine consists of molecules, not individual atoms
When bromine is passed through the mass spectrometer, an electron is given off to give the molecular ion, Br2+
Some of these will fragment to make Br + Br+
-
- Br2+ → Br + Br+
The Br atom passes through the machine, and the Br+ ions will give lines at 79 and 81
There will also be a line for the unfragmented Br2+ ion
This will give 3 molecular ion peaks
-
- Br2+ ion containing the isotopes 79 + 79 = 158
- Br2+containing the isotopes 79 + 81 = 160
- Br2+ containing the isotopes 81 + 81 = 162
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









