- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记4.1.4 Molecular Ion Peak & Fragmentation
Mass Spectrometry: Deducing Molecular Formula
- Each peak in the mass spectrum corresponds to a certain fragment with a particular m/e value
- The peak with the highest m/e value is the molecular ion (M+) peak which gives information about the molecular mass of the compound
- The molecular ion is the entire molecule that has lost one electron when bombarded with a beam of electrons

- The [M+1] peak is a smaller peak which is due to the natural abundance of the isotope carbon-13
- The height of the [M+1] peak for a particular ion depends on how many carbon atoms are present in that molecule; the more carbon atoms, the larger the [M+1] peak is
- For example, the height of the [M+1] peak for an hexane (containing six carbon atoms) ion will be greater than the height of the [M+1] peak of an ethane (containing two carbon atoms) ion
Worked example: Analysing mass spectra

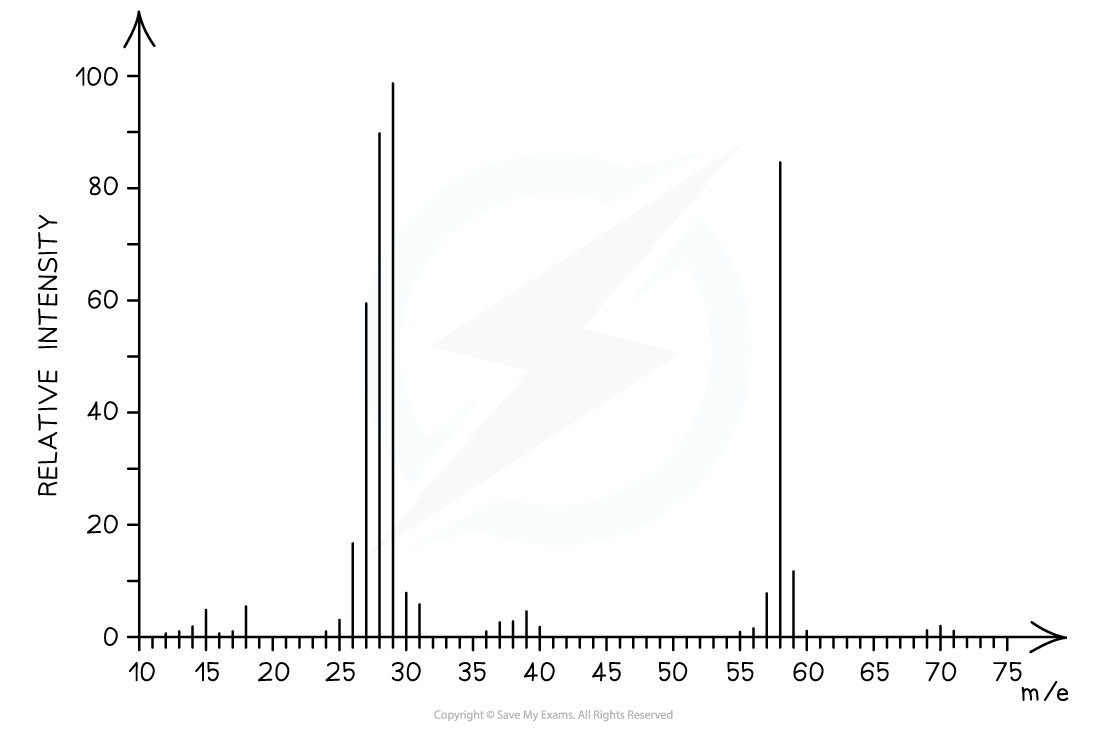
Answer
The mass spectrum corresponds to propanal as the molecular ion peak is at m/e = 58
Propanal arises from the CH3CH2CHO+ ion which has a molecular mass of 58
Butanal arises from the CH3CH2CH2CHO+ ion which has a molecular mass of 72
Identifying Molecules using Fragmentation
- The molecular ion peak can be used to identify the molecular mass of a compound
- However, different compounds may have the same molecular mass
- To further determine the structure of the unknown compound, fragmentation is used
- Fragments may appear due to the formation of characteristic fragments or the loss of small molecules
- For example, a peak at 29 is due to the characteristic fragment C2H5+
- Loss of small molecules give rise to peaks at 18 (H2O), 28 (CO), and 44 (CO2)
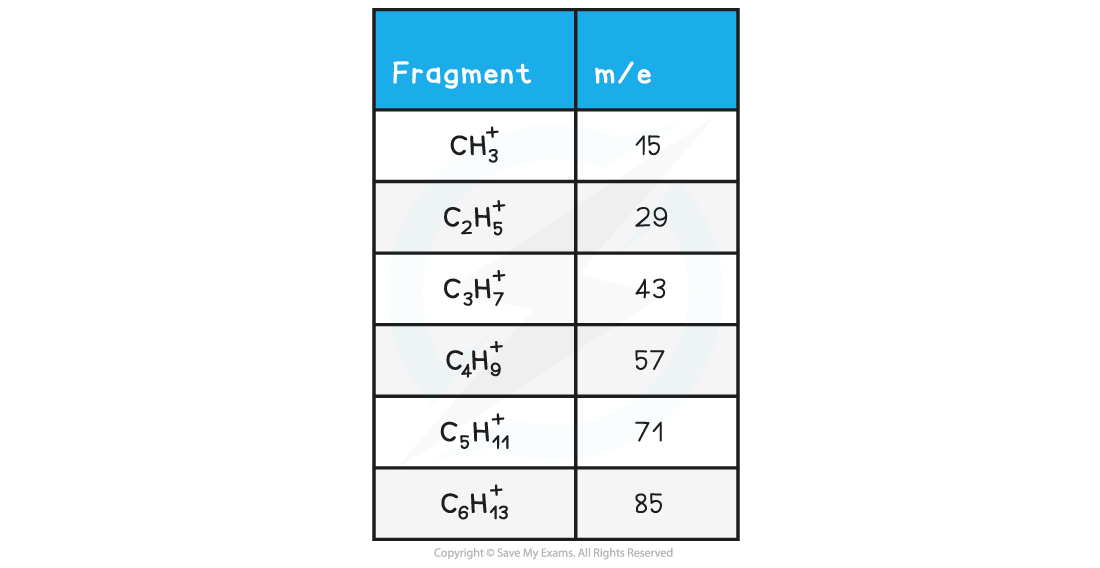
Alkanes
- Simple alkanes are fragmented in mass spectroscopy by breaking the C-C bonds
- M/e values of some of the common alkane fragments are given in the table below
m/e values of fragments table
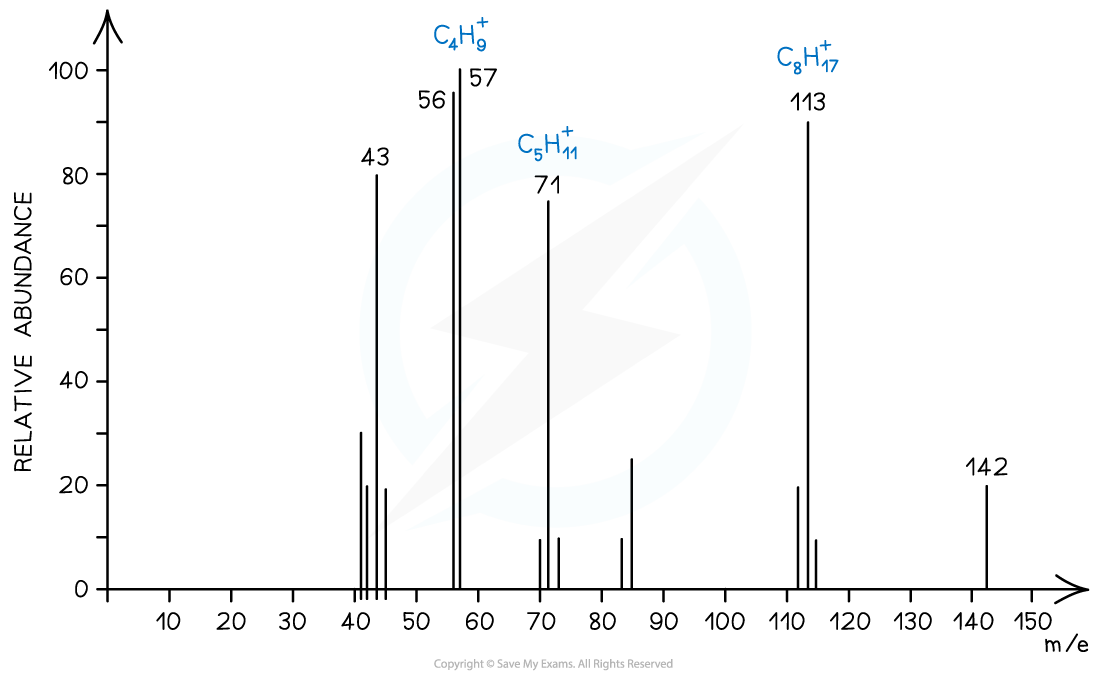
Mass spectrum showing fragmentation of alkanes
Halogenoalkanes
- Halogenoalkanes often have multiple peaks around the molecular ion peak
- This is caused by the fact that there are different isotopes of the halogens
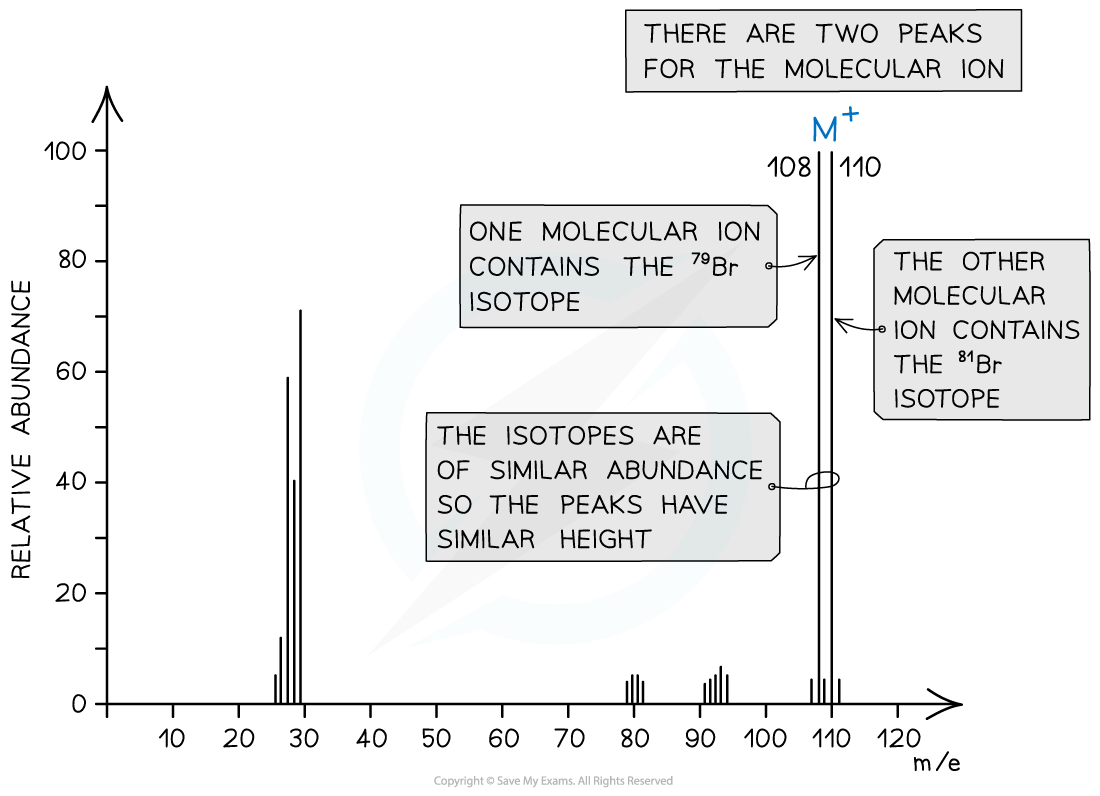
Mass spectrum showing different isotopes of the halogens in the molecular ion
Alcohols
- Alcohols often tend to lose a water molecule giving rise to a peak at 18 below the molecular ion
- Another common peak is found at m/e value 31 which corresponds to the CH2OH+ fragment
- For example, the mass spectrum of propan-1-ol shows that the compound has fragmented in four different ways:
- Loss of H• to form a C3H7O+ fragment with m/e = 59
- Loss of a water molecule to form a C3H6+ fragment with m/e = 42
- Loss of a •C2H5 to form a CH2OH+ fragment with m/e = 31
- And the loss of •CH2OH to form a C2H5+ fragment with m/e = 29
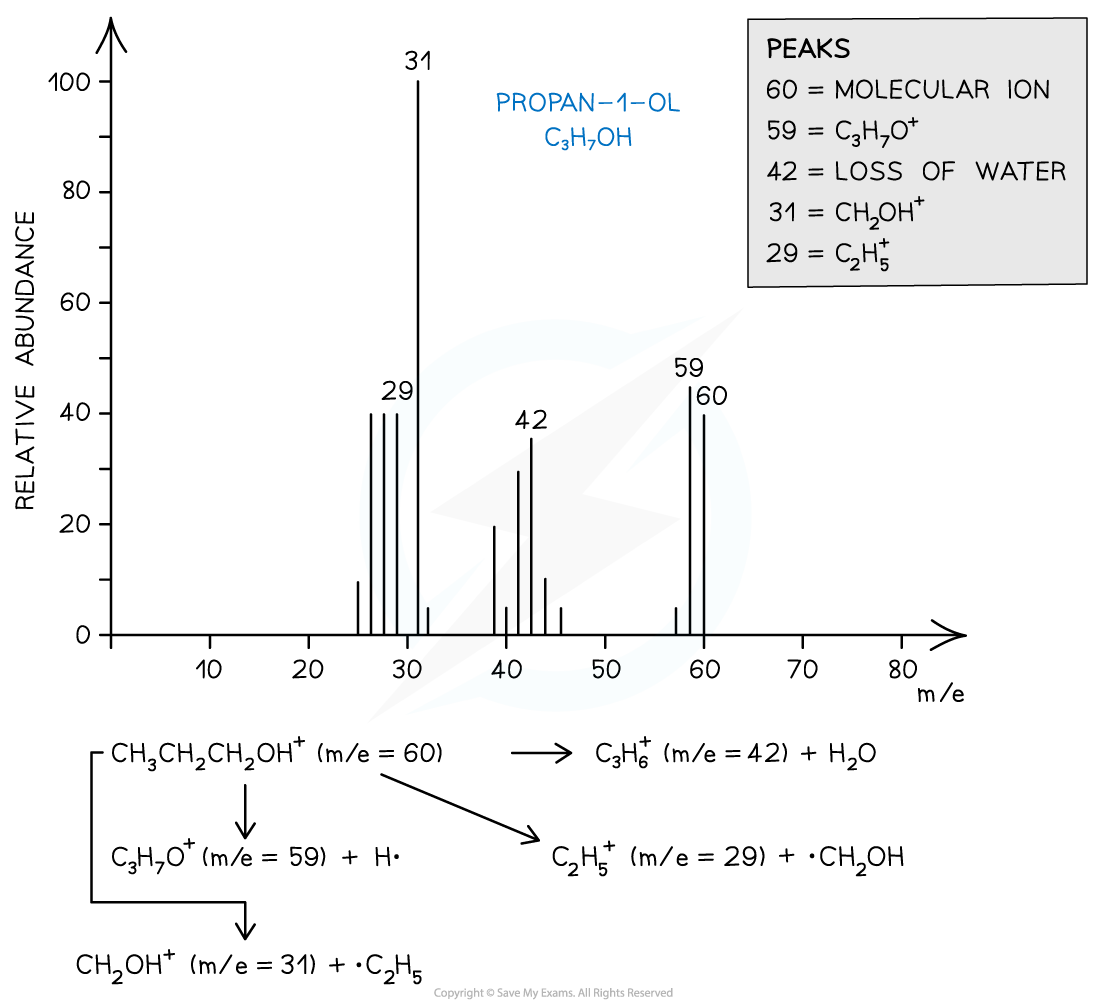
Mass spectrum showing the fragmentation patterns in propan-1-ol (alcohol)
Worked example: Ion fragmentation
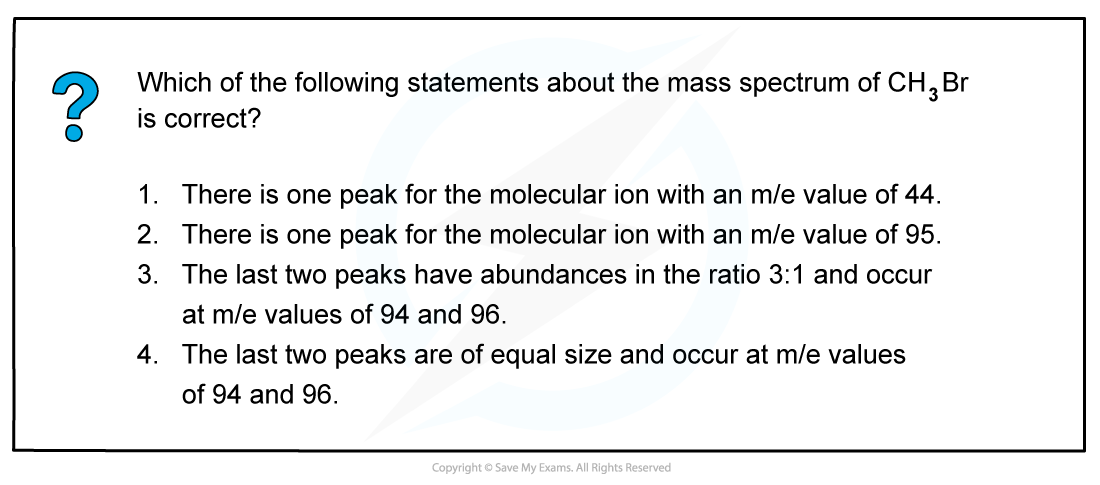
Answer
The correct answer is 4 as bromomethane (CH3Br) will fragment into 3 peaks
-
- CH381Br → [CH381Br]+ + e− at m/e 96
- CH379Br → [CH379Br]+ + e− at m/e 94
- CH3Br → [CH3]+ + •Br at m/e 15
The last two peaks (which correspond to the molecular ion peak) therefore are equal in size and occur at m/e values of 94 and 96
Worked example: Alcohol fragmentation
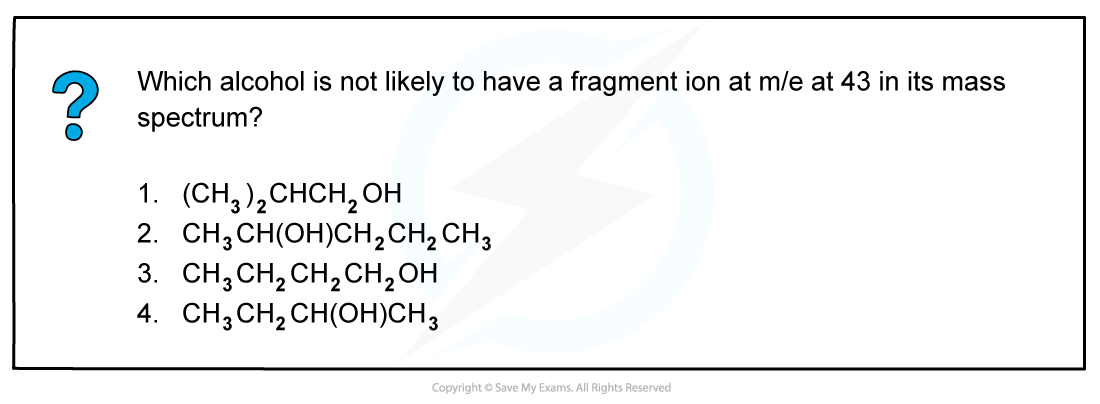
Answer
The correct answer is 4 because a line at m/e = 43 corresponds to an ion with a mass of 43 for example:
-
- [CH3CH2CH2]+
- [(CH3)2CH]+
2-butanol is not likely to have a fragment at m/e = 43 as it does not have either of these fragments in its structure.
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









