- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记3.9.3 Synthetic Routes
Analysis of Synthetic Routes
- Students should be able to critically analyse given synthetic routes and determine whether appropriate reagents and reaction conditions are used
- Students should also be able to predict possible by-product of a synthetic reaction
Worked Example: Two-step synthesis
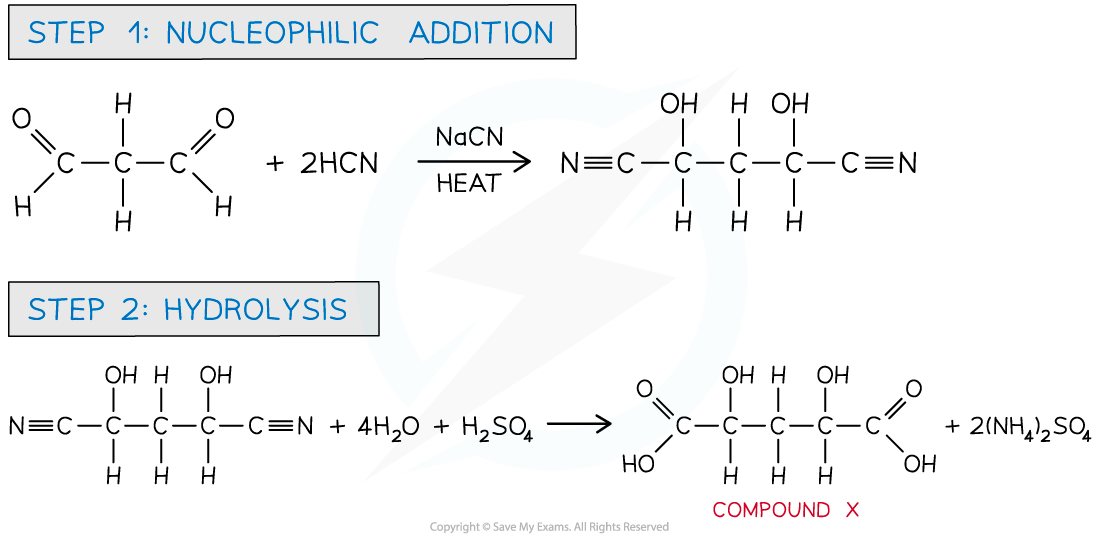
Answer
The correct answer is D
The first step involves a nucleophilic addition of CN- using NaCN as catalyst and heat to form a hydroxynitrile.
In the second step, the nitrile is refluxed with dilute aqueous sulfuric acid causing hydrolysis of the nitrile forming a carboxylic acid and ammonium salt.
Worked Example: Synthesis of hexanoic acid
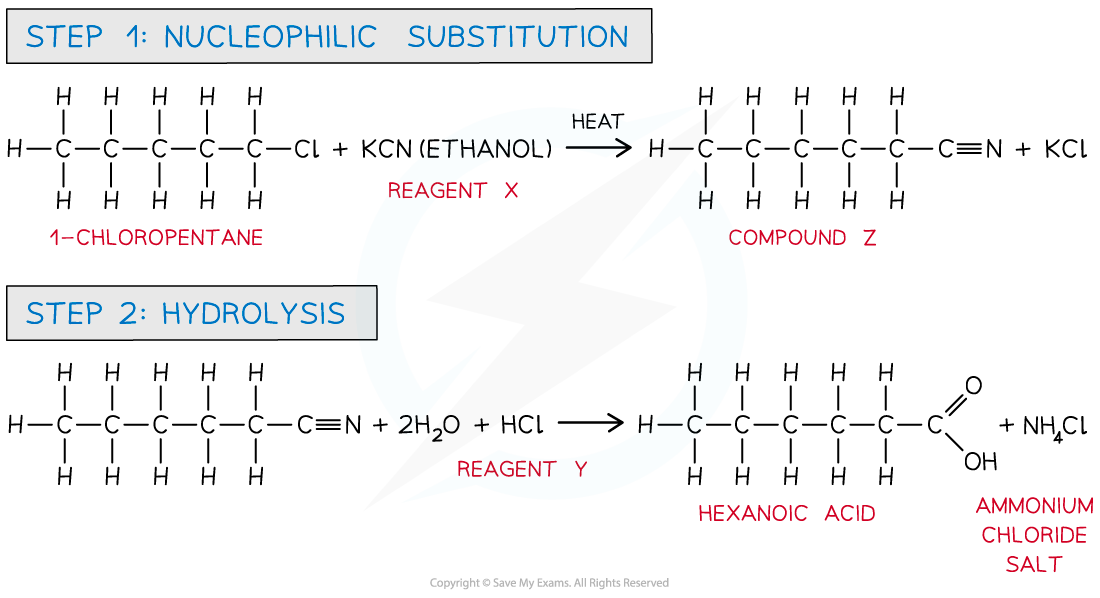
Answer
The correct answer is C
Halogenoalkanes can undergo nucleophilic substitution with ethanolic KCN in which the CN- ion acts as a nucleophile and replaces the chlorine atom in 1-chloropentane to form a nitrile.
The treatment of nitriles with concentrated hydrochloric acid will produce a carboxylic acid and an ammonium salt.
In this case, hexanoic acid and ammonium chloride will be formed.
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









