- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记3.7.2 Nitriles & Hydroxynitriles
Production of Nitriles
- Nitriles are compounds with a -CN functional group
- They can be prepared from the nucleophilic substitution of halogenoalkanes
 Propanenitrile, an example of a nitrile
Propanenitrile, an example of a nitrile
Reaction with KCN
- The nucleophile in this reaction is the cyanide, CN- ion
- Ethanolic solution of potassium cyanide (KCN in ethanol) is heated under reflux with the halogenoalkane
- The product is a nitrile
 Bromoethane reacts with ethanolic potassium cyanide when heated under reflux to form propanenitrile
Bromoethane reacts with ethanolic potassium cyanide when heated under reflux to form propanenitrile
Exam Tip
The nucleophilic substitution of halogenoalkanes with KCN adds an extra carbon atom to the carbon chain.This reaction can therefore be used by chemists to make a compound with one more carbon atom than the best available organic starting material.
Production of Hydroxynitriles
- Hydroxynitriles are compounds with both a hydroxy (-OH) and cyanide (-CN) functional group
- They can be prepared from the nucleophilic addition of aldehydes and ketones
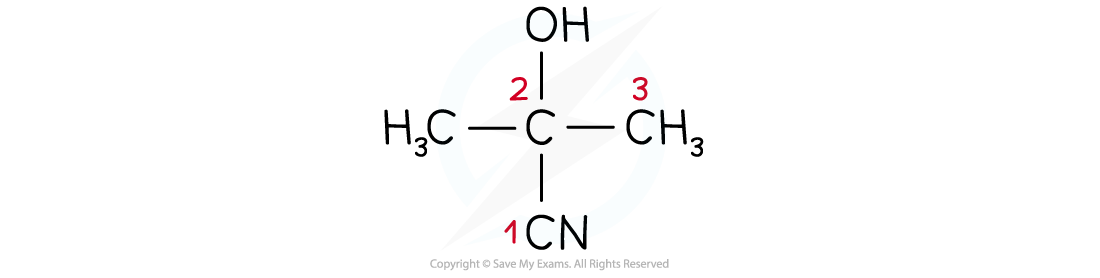 2-hydroxy-2-methylpropanenitrile
2-hydroxy-2-methylpropanenitrile
Reaction with HCN
- The nucleophilic addition of hydrogen cyanide to carbonyl compounds is a two-step process
- In step 1, the cyanide ion attacks the carbonyl carbon to form a negatively charged intermediate
- In step 2, the negatively charged oxygen atom in the reactive intermediate quickly reacts with aqueous H+ (either from HCN, water or dilute acid) to form a 2-hydroxynitrile
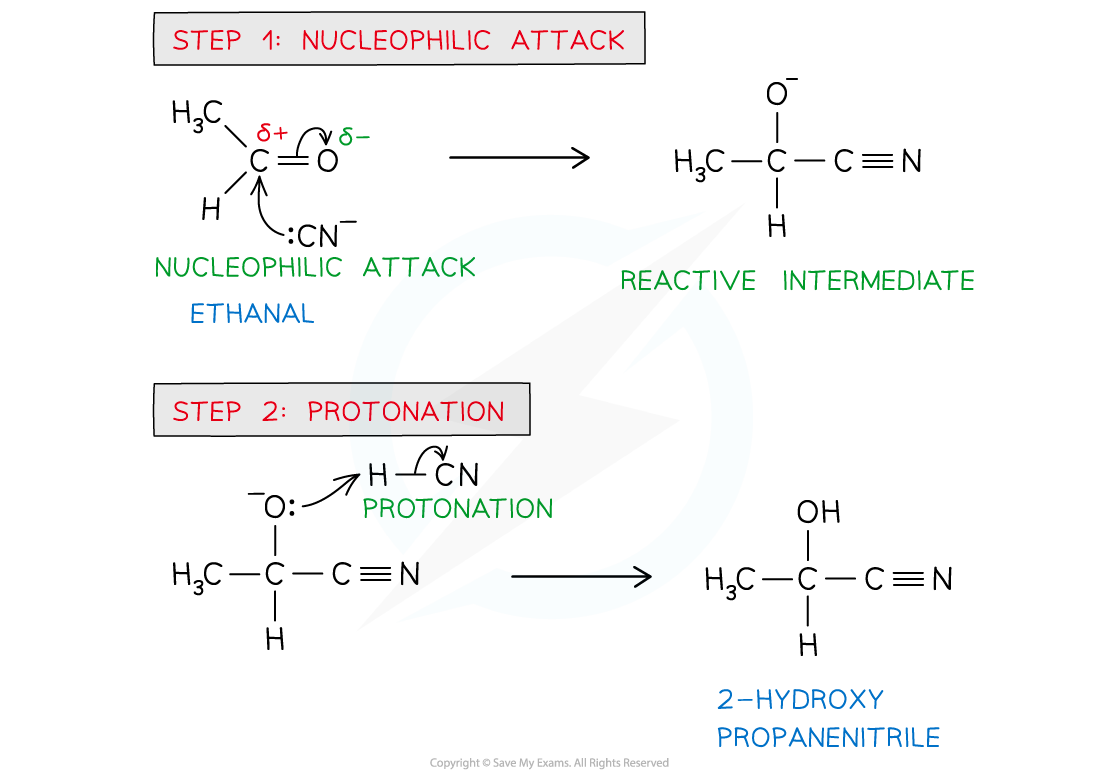 The cyanide ion attacks the carbonyl carbon to form a negatively charged intermediate which quickly reacts with a proton to form a 2-hydroxynitrile compound
The cyanide ion attacks the carbonyl carbon to form a negatively charged intermediate which quickly reacts with a proton to form a 2-hydroxynitrile compound
Exam Tip
The actual negative charge on the cyanide ion is on the carbon atom and not on the nitrogen atom.
Hydrolysis of Nitriles
- Nitriles are hydrolysed by either dilute acid or dilute alkali followed by acidification to give a carboxylic acid
- Hydrolysis is the breakdown of a compound using water
Hydrolysis of nitriles
- Nitriles are hydrolysed by either dilute acid or dilute alkali followed by acidification
- Hydrolysis by dilute acid results in the formation of a carboxylic acid and ammonium salt
- Hydrolysis by dilute alkali results in the formation of a sodium carboxylate salt and ammonia; Acidification is required to change the carboxylate ion into a carboxylic acid
- The -CN group at the end of the hydrocarbon chain is converted to a -COOH group
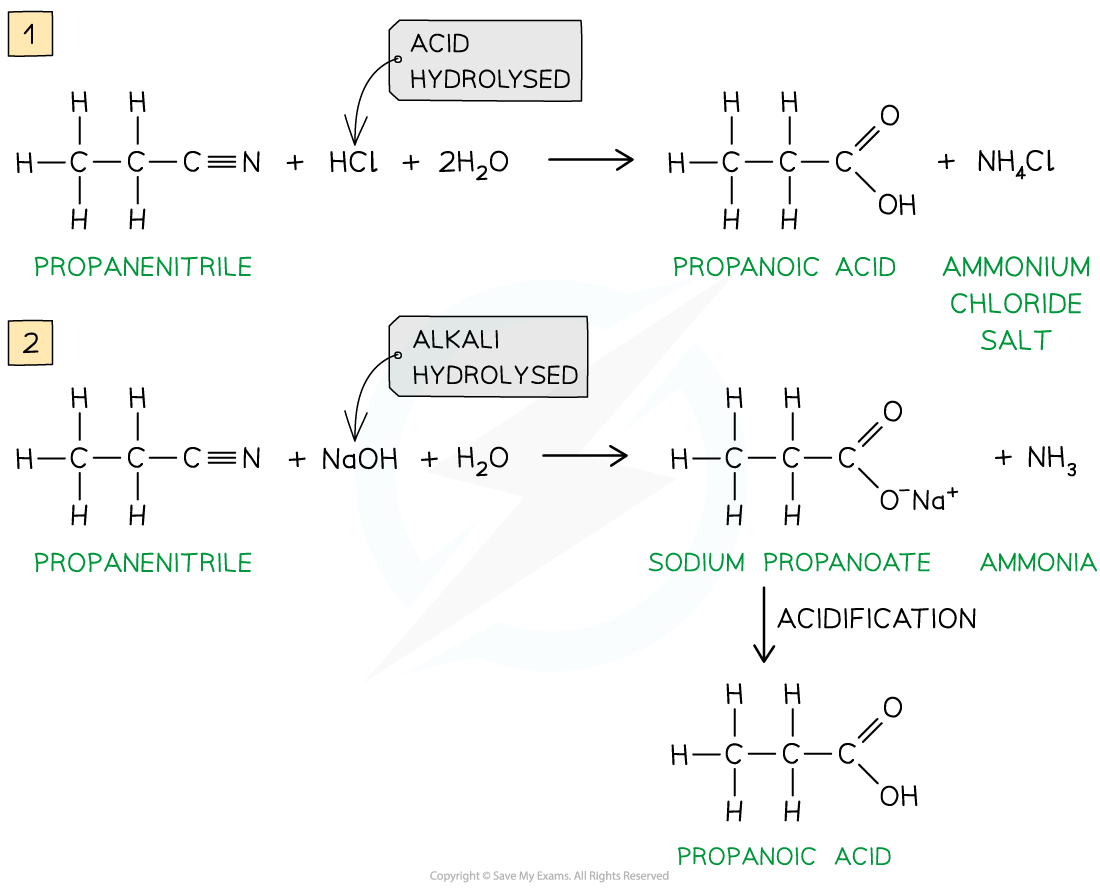
Hydrolysis of nitriles by either dilute acid (1) or dilute alkali and acidification (2) will form a carboxylic acid
Exam Tip
Unlike the formation of nitriles which add an extra carbon atom to the carbon chain, hydrolysis doesn’t change the number of carbon atoms.
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









