- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记3.4.4 Alcohol Dissociation
Acidity of Alcohols
- Alcohols have a low degree of dissociation
- This means, that when dissolved in water, alcohol molecules do not dissociate (split up) to a great extent
ROH (aq) ⇄ RO- (aq) + H+ (aq)
Alcohol alkoxide ion
- The position of the equilibrium lies to the left, meaning that there are far more alcohol molecules than RO- and H+ ions
- When water dissociates, the position of the equilibrium still lies to the left, but there are more H+ ions compared to the dissociation of alcohols
H2O (l) ⇄ OH- (aq) + H+ (aq)
- As alcohols have a lower [H+ (aq)] in solution compared to water, alcohols are weaker acids than water
The inductive effect in alcohols
- Electron-donating species such as alkyl groups push electrons into a covalent bond and are said to have a positive inductive effect
- In alcohols, the oxygen atom in the alkoxide ion is bonded to an electron-donating alkyl group
- This means that there is more electron density on the O- atom
- The alkoxide ion is, therefore, more likely to accept an H+ ion and form the alcohol again
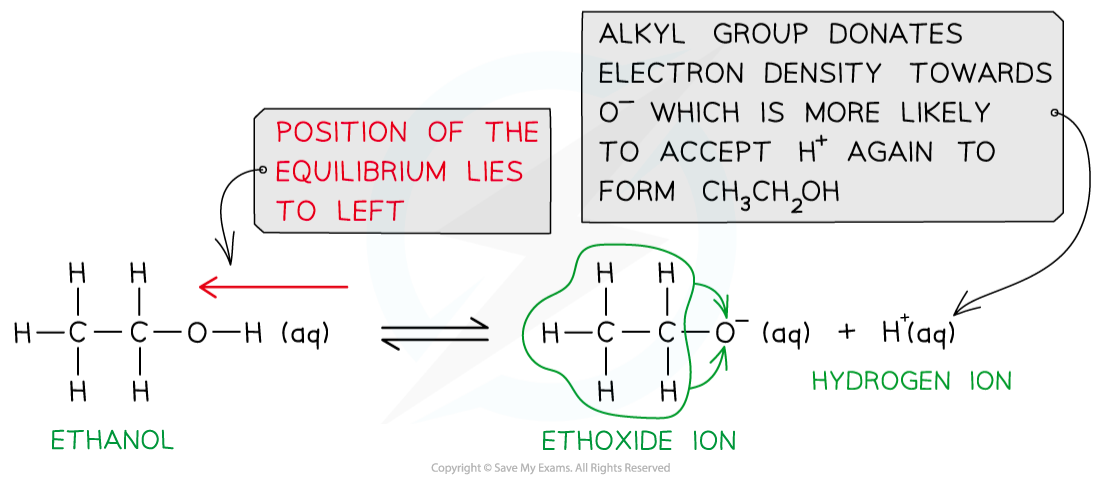 Alkyl groups in the alkoxide ion donate electron density to the negatively charged oxygen, causing it to more readily accept a proton and form the alcohol again
Alkyl groups in the alkoxide ion donate electron density to the negatively charged oxygen, causing it to more readily accept a proton and form the alcohol again
- When water dissociates, the hydroxide ion only has one other hydrogen atom
- There is no extra electron density on the oxygen which is less likely to accept an H+ ion
- Water is therefore a stronger acid than alcohols
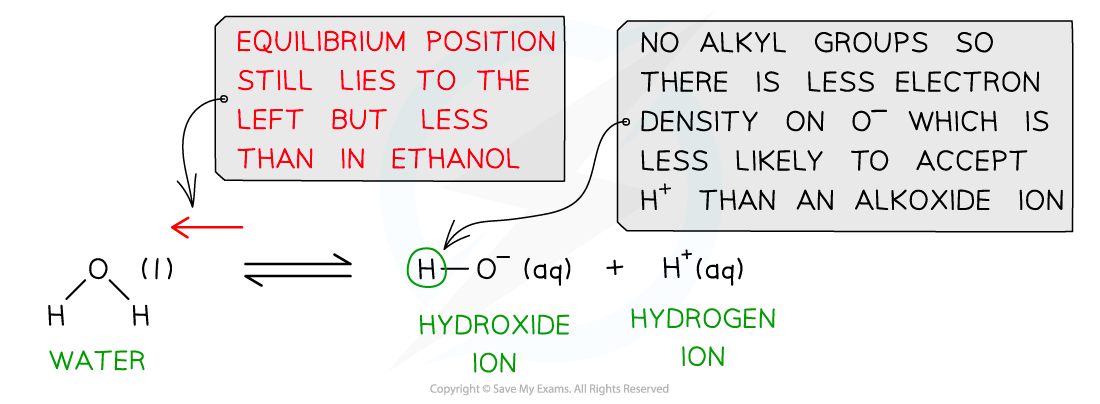 Water is a stronger acid than alcohols as there are no electron-donating groups in the hydroxide ion, causing the O- to be less likely to accept a proton and reform water
Water is a stronger acid than alcohols as there are no electron-donating groups in the hydroxide ion, causing the O- to be less likely to accept a proton and reform water
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








