- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记3.4.2 Reactions of Alcohols
Reactions of Alcohols
- Alcohols are reactive molecules which undergo a wide range of reactions
Combustion of alcohols
- Alcohols react with oxygen in the air when ignited and undergo complete combustion to form carbon dioxide and water
Alcohol + oxygen → carbon dioxide + water
 Complete combustion of alcohols to produce carbon dioxide and water
Complete combustion of alcohols to produce carbon dioxide and water
Substitution of alcohols
- In the substitution of alcohols, a hydroxy group (-OH) is replaced by a halogen to form an halogenoalkane
- The substitution of the alcohol group for a halogen can be achieved by reacting the alcohol with:
- HX (rather than using HBr, KBr is reacted with H2SO4 or H3PO4 to make HBr that will then react with the alcohol)
- PCl3 and heat
- PCl5 at room temperature
- SOCl2
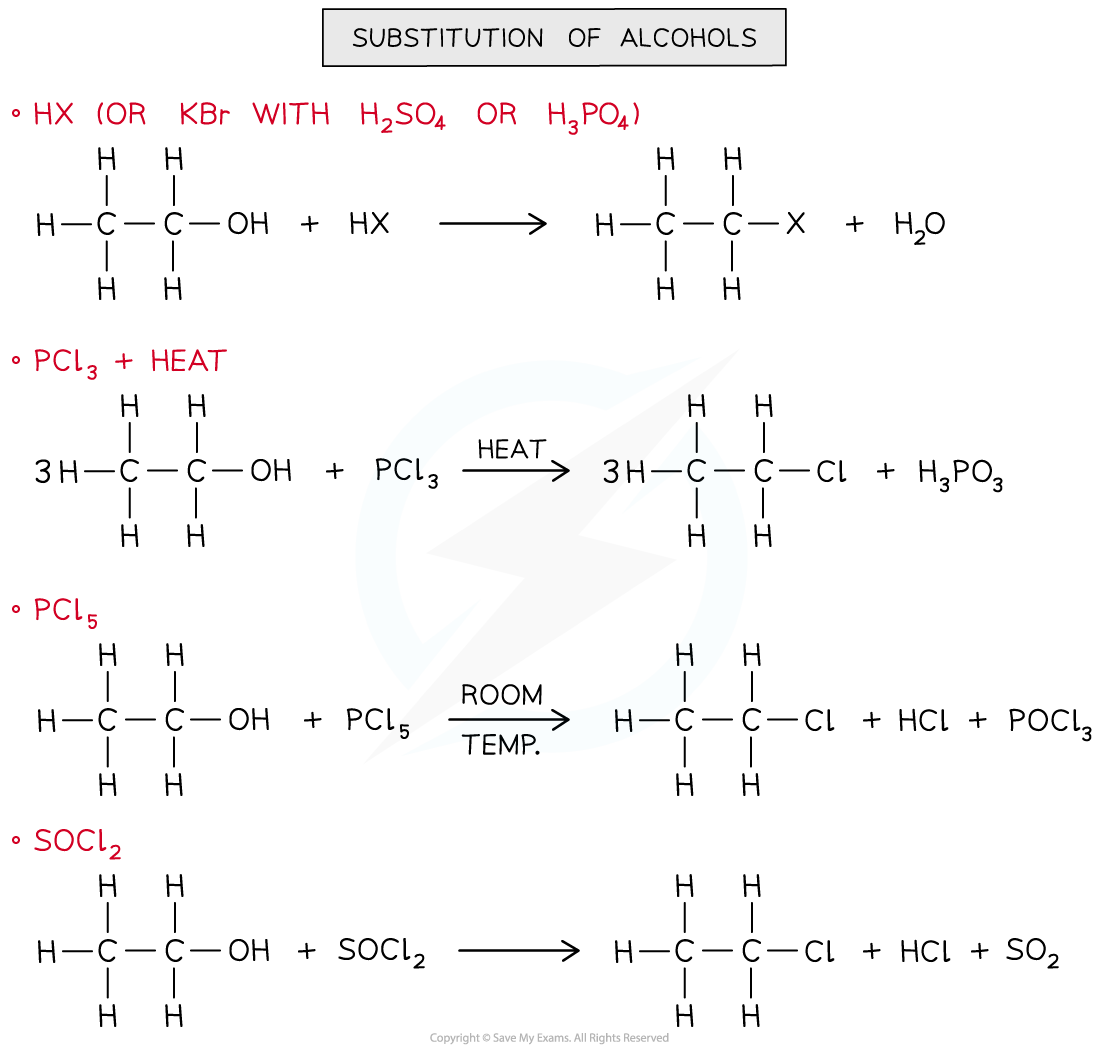 Substitution of alcohols to produce halogenoalkanes
Substitution of alcohols to produce halogenoalkanes
Reaction with Na
- When an alcohol reacts with a reactive metal such as sodium (Na), the oxygen-hydrogen bond in the hydroxy group breaks
- Though the reaction is less vigorous than sodium reacting with water, hydrogen gas is given off and a basic compound (alkoxide) is formed
- If the excess ethanol is evaporated off after the reaction a white crystalline solid of sodium alkoxide is left
Alcohol + sodium → sodium alkoxide + hydrogen
- The longer the hydrocarbon chain in the alcohol, the less vigorous the reaction becomes
 Alcohols react with Na to form a basic sodium alkoxide salt and hydrogen gas
Alcohols react with Na to form a basic sodium alkoxide salt and hydrogen gas
Oxidation of alcohols
- Primary alcohols can be oxidised to form aldehydes which can undergo further oxidation to form carboxylic acids
- Secondary alcohols can be oxidised to form ketones only
- Tertiary alcohols do not undergo oxidation
- The oxidising agents of alcohols include acidified K2Cr2O7 or acidified KMnO4
- Acidified potassium dichromate(VI), K2Cr2O7, is an orange oxidising agent
- Acidified means that that the potassium dichromate(VI) is in a solution of dilute acid (such as dilute sulfuric acid)
- For potassium dichromate(VI) to act as an oxidising agent, it itself needs to be reduced
- This reduction requires hydrogen (H+) ions which are provided by the acidic medium
- When alcohols are oxidised the orange dichromate ions (Cr2O72-) are reduced to green Cr3+ ions
- Acidified potassium manganate(VII), KMnO4, is a purple oxidising agent
- As with acidified K2Cr2O7 the potassium manganate(VII) is in an acidic medium to allow reduction of potassium manganate(VII) to take place
- When alcohols are oxidised, the purple manganate ions (MnO4-) are reduced to colourless Mn2+ ions
- As with acidified K2Cr2O7 the potassium manganate(VII) is in an acidic medium to allow reduction of potassium manganate(VII) to take place
- Warm primary alcohol is added to the oxidising agent
- The formed aldehyde has a lower boiling point than the alcohol reactant so it can be distilled off as soon as it forms
- If the aldehyde is not distilled off, further refluxing with excess oxidising agent will oxidise it to a carboxylic acid
- Since ketones cannot be further oxidised, the ketone product does not need to be distilled off straight away after it has been formed
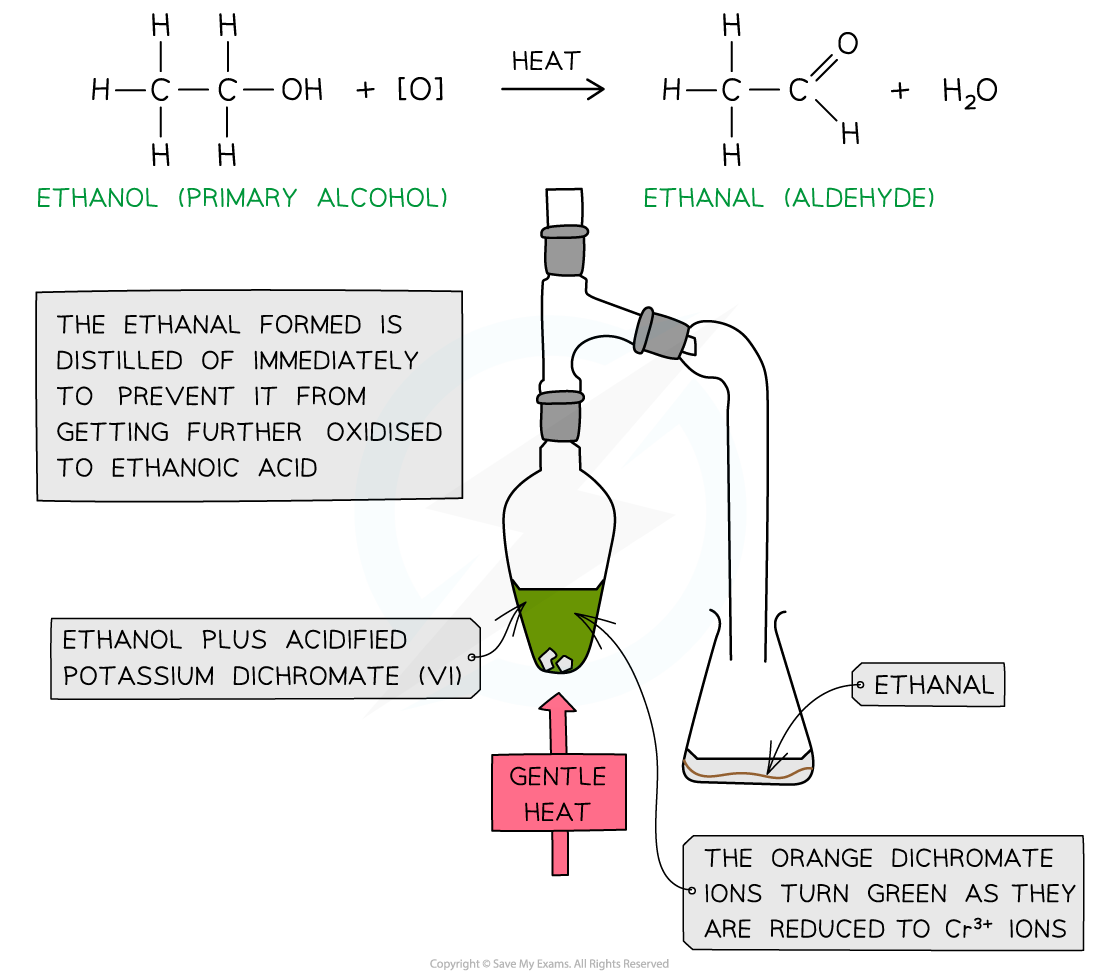
Oxidation of ethanol by acidified K2Cr2O7 to form an aldehyde by distillation and carboxylic acid by refluxing

Oxidation of propan-2-ol by acidified K2Cr2O7 to form a ketone by distillation
Dehydration of alcohols
- Alcohols can also undergo dehydration to form alkenes
- Dehydration is a reaction in which a water molecule is removed from a larger molecule
- A dehydration reaction is a type of elimination reaction
- Alcohol vapour is passed over a hot catalyst of aluminium oxide (Al2O3) powder OR pieces of porous pot or pumice as well as concentrated acid can be used as catalysts
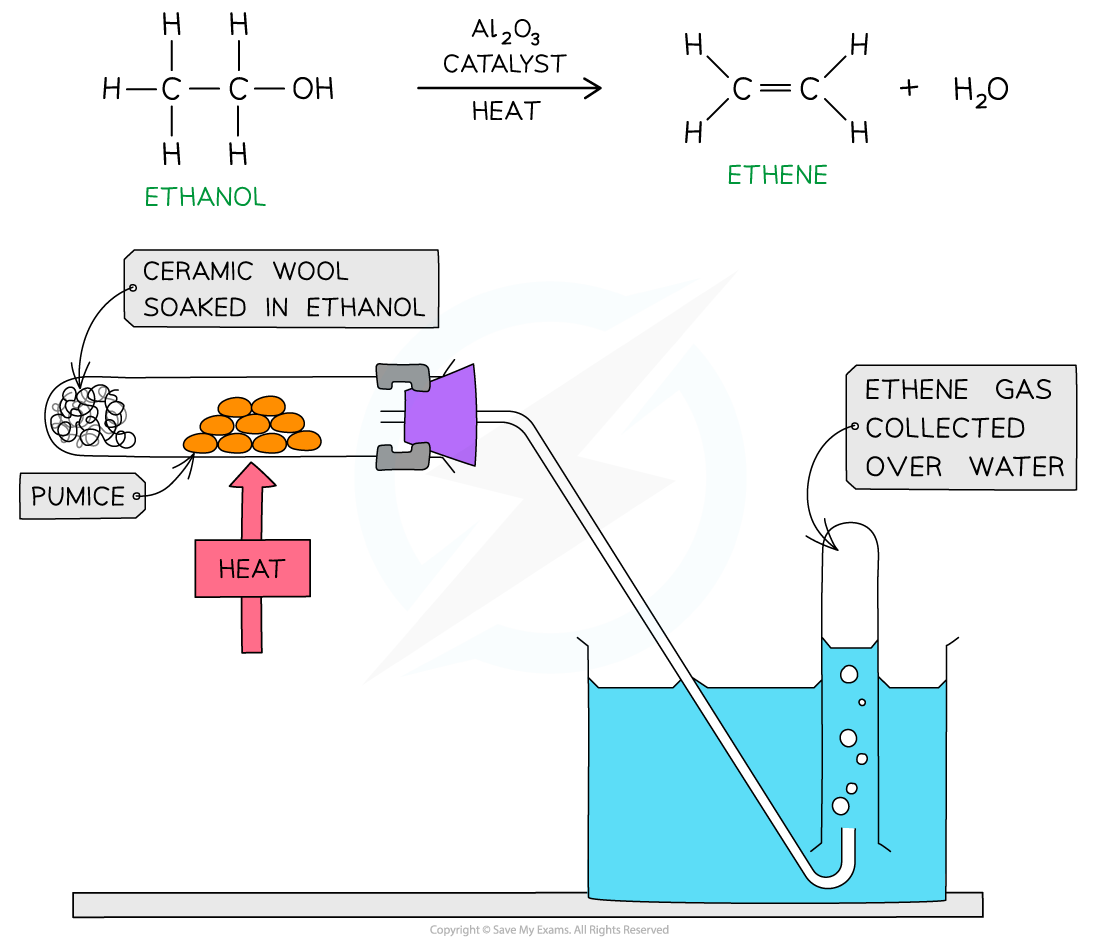
Dehydration of ethanol using aluminium oxide as a catalyst forms ethene gas, which can be collected over water
Esterification of Alcohols
- Esterification is a condensation reaction between a carboxylic acid and an alcohol to form an ester and a water molecule
- For esterification to take place, the carboxylic acid and alcohol are heated under reflux with a strong acid catalyst (such as H2SO4 or H3PO4)
Carboxylic acid + alcohol → ester + water
- The reaction is reversible so an equilibrium mixture can be established with all the reactants and products
- Esters have sweet, fruity smells
 Esterification of ethanol and ethanoic acid using a strong acid catalyst to form ethyl ethanoate and water
Esterification of ethanol and ethanoic acid using a strong acid catalyst to form ethyl ethanoate and water
Exam Tip
The first part of an ester’s name comes from the alcohol, whereas the second part comes from the carboxylic acid.So, if ethanol and propanoic acid react together, this will make the ester ethyl propanoate.
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









