- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记3.4.1 Production of Alcohols
Production of Alcohols
- Alcohols are compounds that contain at least one hydroxy (-OH) group
- The general formula of alcohols is CnH2n+1OH
- Alcohols can be prepared by a wide range of chemical reactions
Electrophilic addition of alkenes
- When hot steam is reacted with an alkene, using concentrated phosphoric(VI) acid (H3PO4)as a catalyst, electrophilic addition takes place to form an alcohol

Electrophilic addition of steam to alkenes to form an alcohol
Oxidation of alkenes
- Cold, dilute KMnO4 is a mild oxidising agent and oxidises alkenes
- The C-C double bond is not fully broken and a diol is formed
- A diol is a compound with two hydroxy, -OH, groups

Oxidation of alkenes using cold, dilute KMnO4 to form a diol
Nucleophilic substitution of halogenoalkanes
- The halide atom in halogenoalkanes can be substituted when heated with aqueous NaOH in a nucleophilic substitution reaction
 Nucleophilic substitution of halogenoalkanes using NaOH to form an alcohol
Nucleophilic substitution of halogenoalkanes using NaOH to form an alcohol
Reduction of aldehyde & ketones
- Aldehydes and ketones can be reduced by reducing agents such as NaBH4 or LiAlH4
- Aldehydes are reduced to primary alcohols
- The carbon attached to the hydroxy group is bonded to one other alkyl group
- Ketones are reduced to secondary alcohols
- The carbon attached to the hydroxy group is bonded to two other alkyl groups
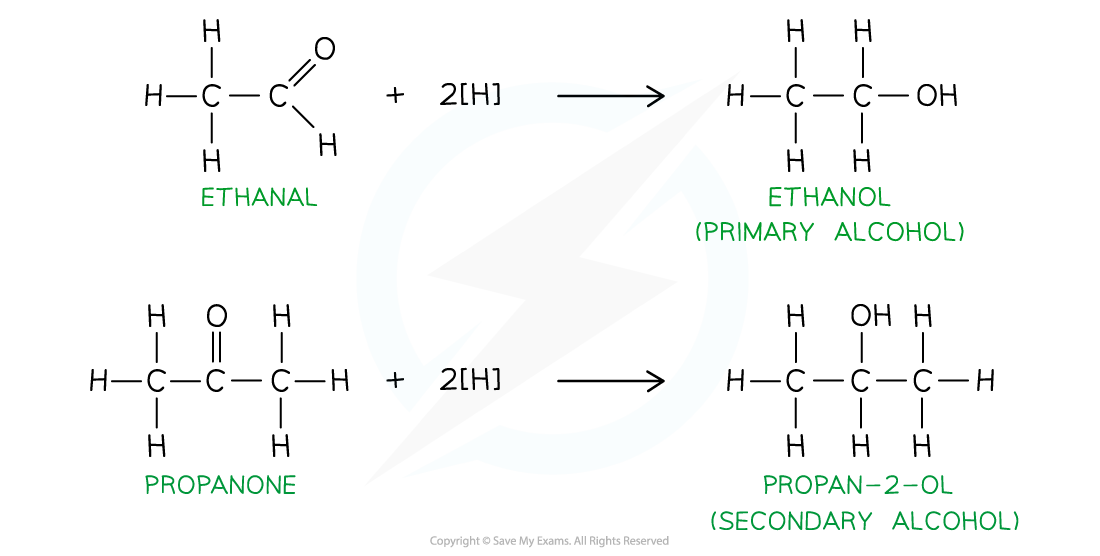 Reduction of aldehydes and ketones to form primary and secondary alcohols
Reduction of aldehydes and ketones to form primary and secondary alcohols
Reduction of carboxylic acids
- Similarly, carboxylic acids are reduced by NaBH4 or LiAlH4 to primary alcohols
- Carboxylic acids can also be reduced by H2 using a nickel catalyst and heat

Reduction of carboxylic acids to primary alcohols
Hydrolysis of ester
- Esters are made by a condensation reaction between an alcohol and a carboxylic acid
- When an ester is heated with dilute acid or alkali, hydrolysis will take place and the carboxylic acid and alcohol will be reformed
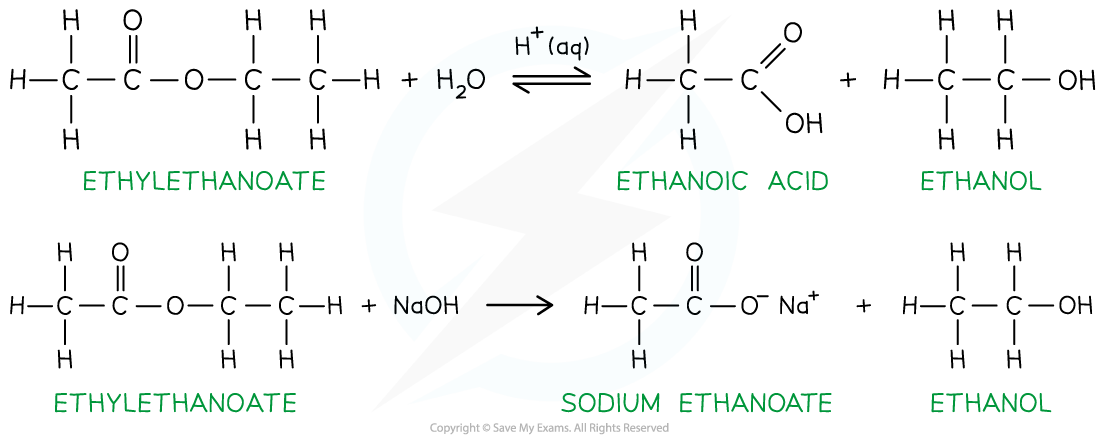
Hydrolysis of esters to form alcohols
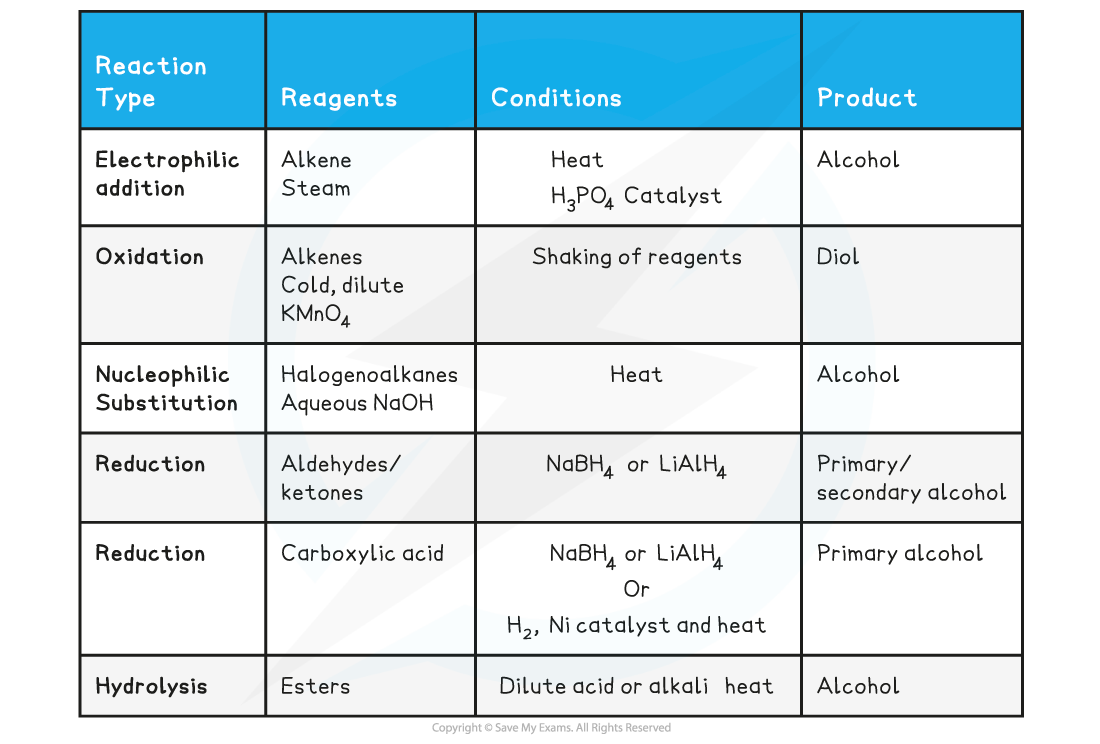
Alcohol production reactions table
Exam Tip
The symbol [O] is used to represent oxygen provided by an oxidising agent.
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








