- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记3.3.2 Substitution Reactions of Halogenoalkanes
Nucleophilic Substitution Reactions of Halogenoalkanes
- Halogenoalkanes are much more reactive than alkanes due to the presence of the electronegative halogens
- The halogen-carbon bond is polar causing the carbon to carry a partial positive and the halogen a partial negative charge
- A nucleophilic substitution reaction is one in which a nucleophile attacks a carbon atom which carries a partial positive charge
- An atom that has a partial negative charge is replaced by the nucleophile
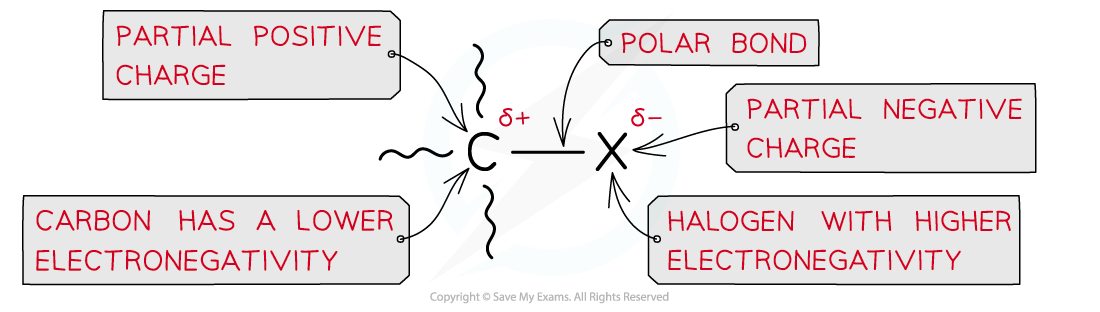 Due to large differences in electronegativity between the carbon and halogen atom, the C-X bond is polar
Due to large differences in electronegativity between the carbon and halogen atom, the C-X bond is polar
Reaction with NaOH
- The reaction of a halogenoalkane with aqueous alkali results in the formation of an alcohol
- The halogen is replaced by the OH-
- The aqueous hydroxide (OH- ion) behaves as a nucleophile by donating a pair of electrons to the carbon atom bonded to the halogen
- Hence, this reaction is a nucleophilic substitution
- For example, bromoethane reacts with aqueous alkali when heated to form ethanol
 The halogen is replaced by a nucleophile, OH-
The halogen is replaced by a nucleophile, OH-
Reaction with KCN
- The nucleophile in this reaction is the cyanide, CN- ion
- Ethanolic solution of potassium cyanide (KCN in ethanol) is heated under reflux with the halogenoalkane
- The product is a nitrile
- For example, bromoethane reacts with ethanolic potassium cyanide when heated under reflux to form propanenitrile
 The halogen is replaced by a cyanide group, CN-
The halogen is replaced by a cyanide group, CN-
- The nucleophilic substitution of halogenoalkanes with KCN adds an extra carbon atom to the carbon chain
- This reaction can therefore be used by chemists to make a compound with one more carbon atom than the best available organic starting material
Reaction with NH3
- The nucleophile in this reaction is the ammonia, NH3 molecule
- An ethanolic solution of excess ammonia (NH3 in ethanol) is heated under pressure with the halogenoalkane
- The product is a primary amine
- For example, bromoethane reacts with excess ethanolic ammonia when heated under pressure to form ethylamine
 The halogen is replaced by an amine group, NH3
The halogen is replaced by an amine group, NH3
- It is very important that the ammonia is in excess as the product of the nucleophilic substitution reaction, the ethylamine, can act as a nucleophile and attack another bromoethane to form the secondary amine, diethylamine
Reaction with aqueous silver nitrate
- Halogenoalkanes can be broken down under reflux by water to form alcohols
- The breakdown of a substance by water is also called hydrolysis
- This reaction is classified as a nucleophilic substitution reaction with water molecules in aqueous silver nitrate solution acting as nucleophiles, replacing the halogen in the halogenoalkane
- For example, bromoethane reacts with aqueous silver nitrate solution to form ethanol
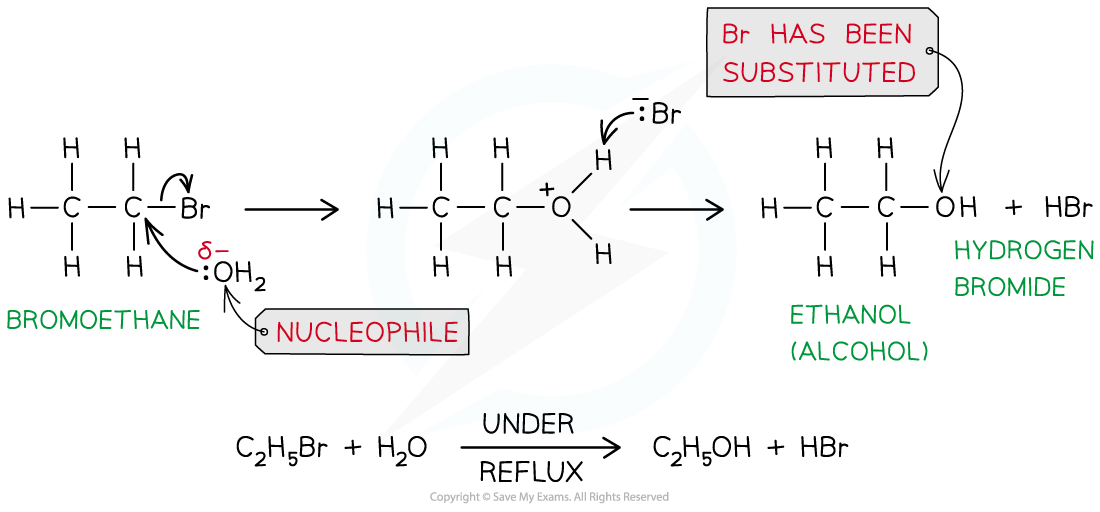 The halogen is replaced by a hydroxyl group, OH-
The halogen is replaced by a hydroxyl group, OH-
- This reaction is similar to the nucleophilic substitution reaction of halogenoalkanes with aqueous alkali, however, hydrolysis with water is much slower than with the OH- ion in alkalis
- The hydroxide ion is a better nucleophile than water as it carries a full formal negative charge
- In water, the oxygen atom only carries a partial negative charge
 A hydroxide ion is a better nucleophile as it has a full formal negative charge whereas the oxygen atom in water only carries a partial negative charge; this causes the nucleophilic substitution reaction with water to be much slower than with aqueous alkali
A hydroxide ion is a better nucleophile as it has a full formal negative charge whereas the oxygen atom in water only carries a partial negative charge; this causes the nucleophilic substitution reaction with water to be much slower than with aqueous alkali
- The halogenoalkanes have different rates of hydrolysis, so this reaction can be used as a test to identify halogens in a halogenoalkane by measuring how long it takes for the test tubes containing the halogenoalkane and aqueous silver nitrate solutions to become opaque
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








