- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记3.2.7 Production of Alkenes
Production of Alkenes: Elimination, & Dehydration Reactions & Cracking
- Alkenes can be made by a series of reactions including elimination, dehydration reactions and cracking
Elimination reaction
- Alkenes can be produced from the elimination reaction of a halogenoalkane
- An elimination reaction is one in which a small molecule is lost
- In the case of halogenoalkanes, the small molecule that is eliminated is a hydrogen halide, HX, where X is the halogen
- The halogenoalkane is heated with ethanolic sodium hydroxide
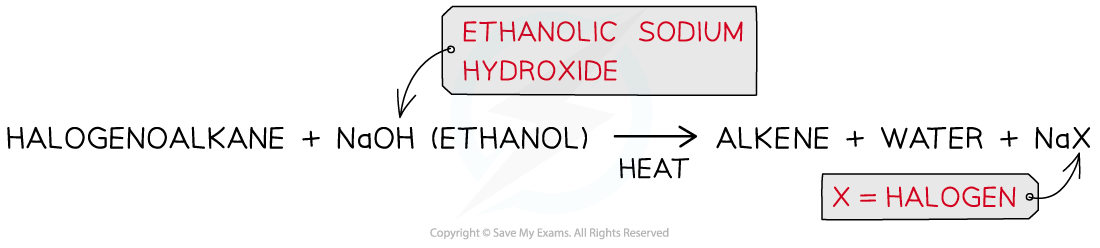
Production of an alkene from a halogenoalkane by reacting it with ethanolic sodium hydroxide and heating it
- The eliminated H+ in HBr reacts with the ethanolic OH- to form water
- The eliminated Br- in HBr reacts with Na+ to form NaBr
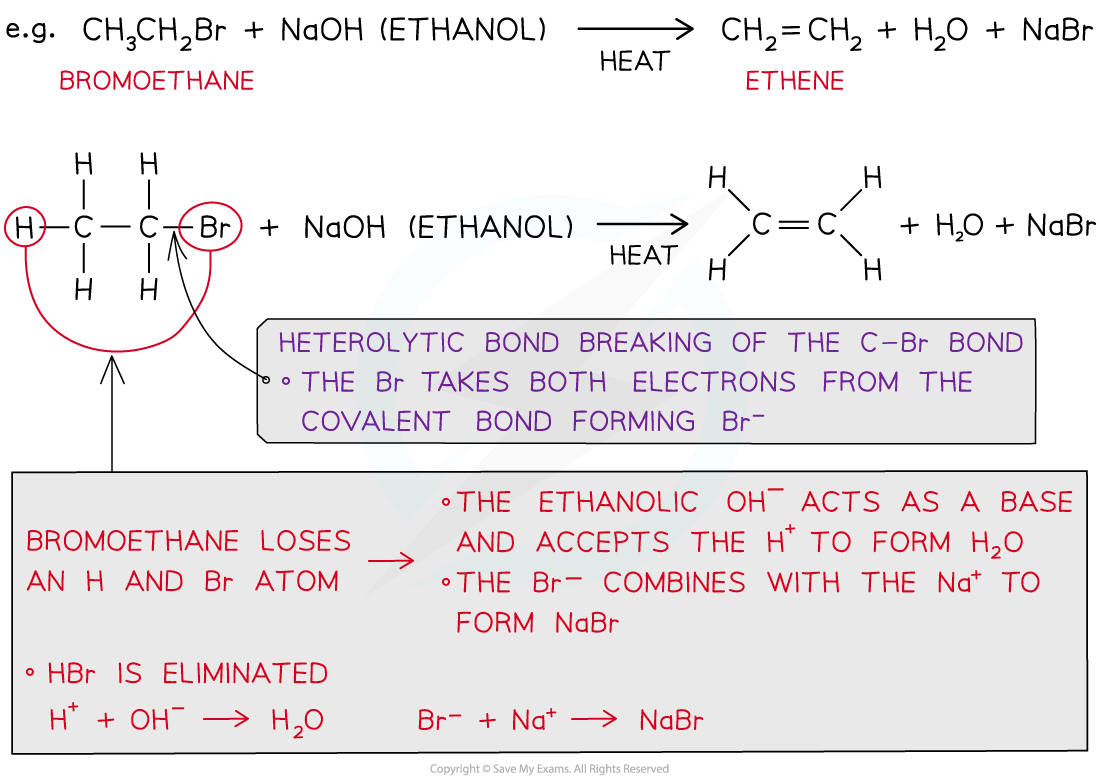 The eliminated HBr reacts with ethanolic OH- and Na+ to form H2O and NaBr
The eliminated HBr reacts with ethanolic OH- and Na+ to form H2O and NaBr
- Note that the reaction conditions should be stated correctly as different reaction conditions will result in different types of organic reactions
- NaOH (ethanol): an elimination reaction occurs to form an alkene
- NaOH (aq): a nucleophilic substitution reaction occurs, and an alcohol is one of the products
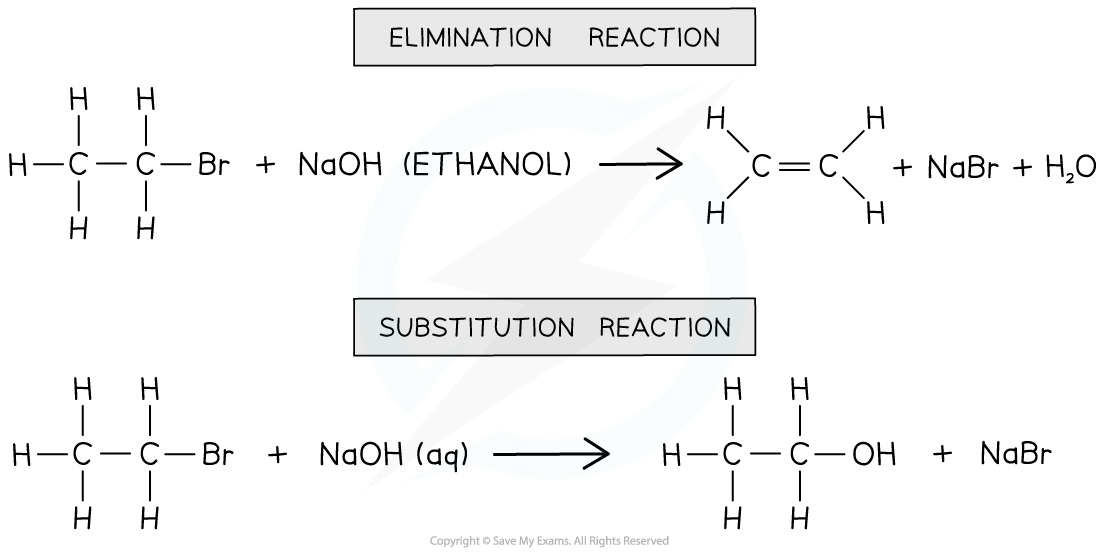
Different reaction conditions will give different products
Dehydration reaction
- Alkenes can also be produced from the elimination reaction of alcohols in which a water molecule is lost
- This is also called a dehydration reaction
- Alcohol vapour is passed over a hot catalyst of aluminium oxide powder (Al2O3)
- Concentrated acid, pieces of porous pot or pumice can also be used as catalysts

Production of an alkene from an alcohol by using a hot aluminium oxide powder catalyst
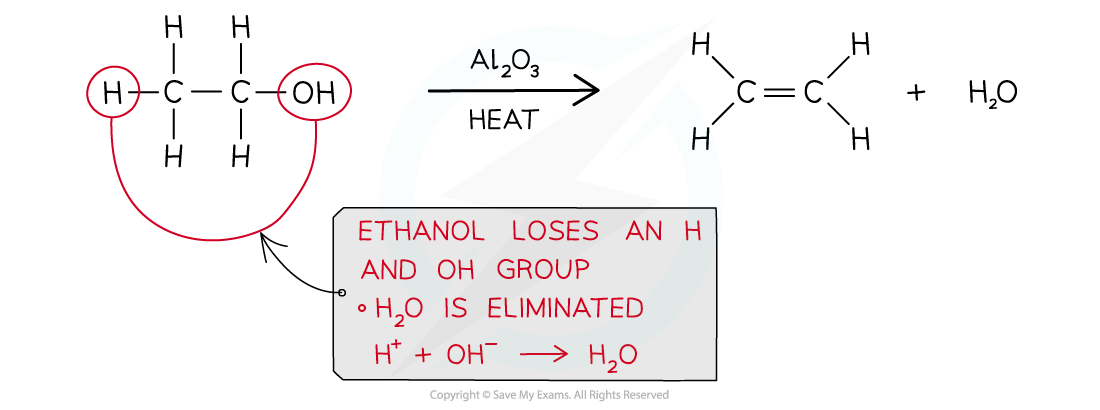
The formation of ethene from ethanol is an example of a dehydration reaction of alcohols
- The smaller alkenes (such as ethene, propene and butene) are all gases at room temperature and can be collected over water
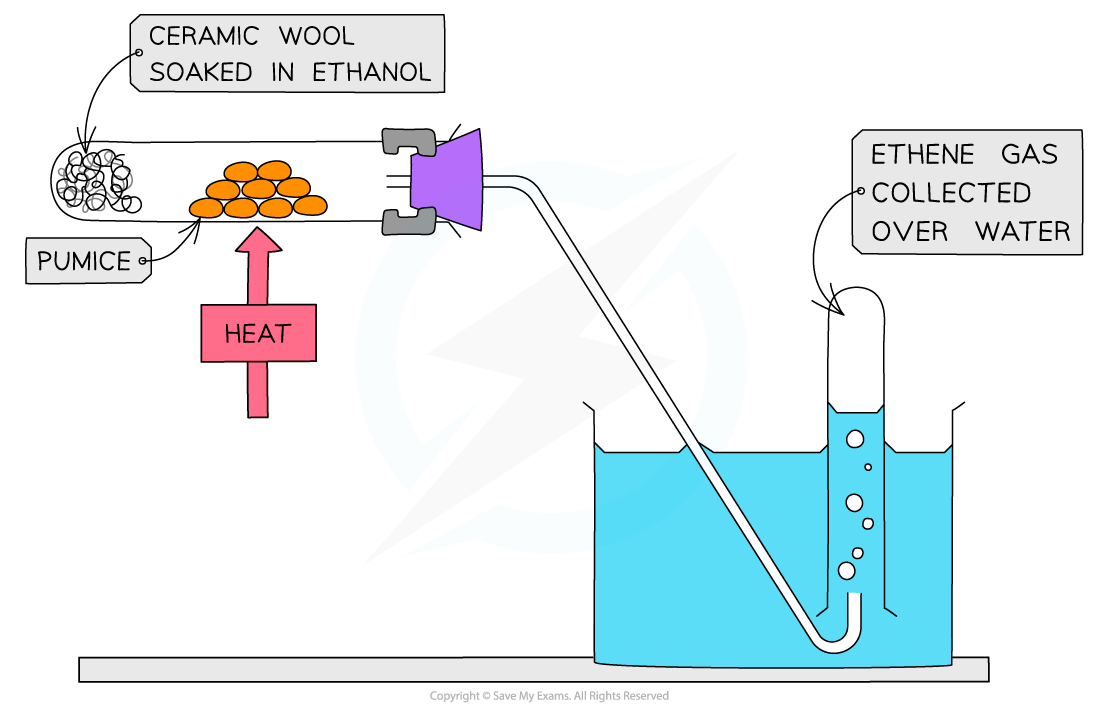 The smaller alkenes are gases at room temperature and collected over water
The smaller alkenes are gases at room temperature and collected over water
Cracking
- Alkenes can also be produced from the cracking of long hydrocarbon molecules in crude oil
- An aluminium oxide (Al2O3) catalyst and high temperatures are used to speed up this reaction.
- It is important to ensure that the crude oil doesn’t come into contact with oxygen as this can cause combustion of the hydrocarbons to produce water and carbon dioxide
- The cracking of crude oil produces a smaller alkane and alkene molecules
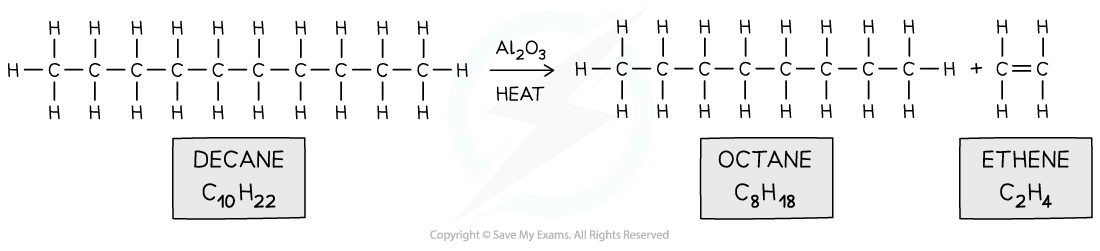
Long hydrocarbon fraction is cracked into two smaller ones
- The low-molecular mass alkenes are more reactive than alkanes as they have an electron-rich double bond
- They can therefore be used as feedstock for making new products
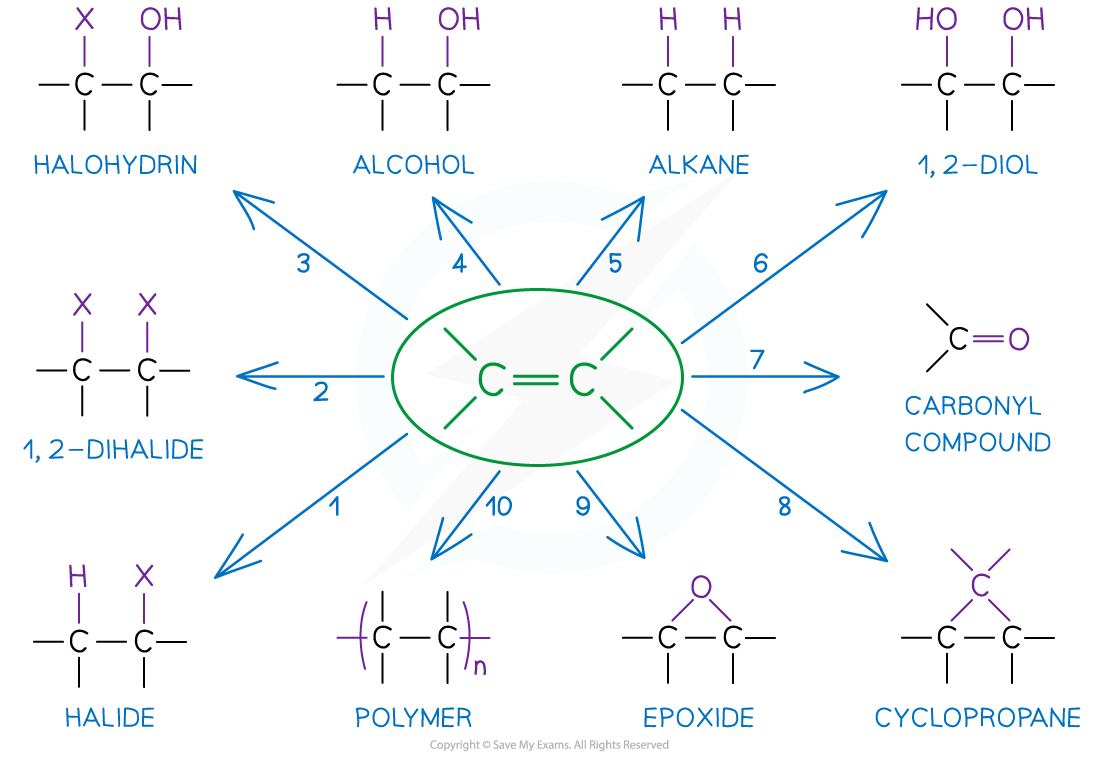 Alkenes are reactive molecules and can undergo many different types of reactions making them useful as starting compounds
Alkenes are reactive molecules and can undergo many different types of reactions making them useful as starting compounds
转载自savemyexams


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








