- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记3.2.2 Combustion & Free Radical Substitution of Alkanes
Combustion & Free Radical Substitution of Alkanes
- Alkanes are combusted (burnt) on a large scale for their use as fuels
- They also react in free-radical substitution reactions to form more reactive halogenoalkanes
Complete combustion
- When alkanes are burnt in excess (plenty of) oxygen, complete combustion will take place and all carbon and hydrogen will be oxidised to carbon dioxide and water respectively
- For example, the complete combustion of octane to carbon dioxide and water
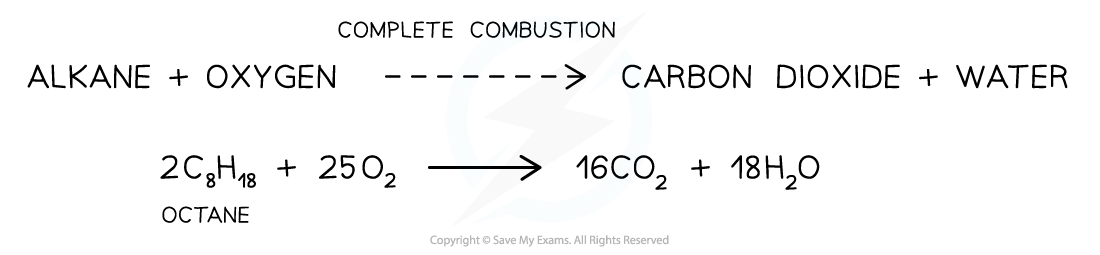
The complete combustion of alkanes
Incomplete combustion
- When alkanes are burnt in only a limited supply of oxygen, incomplete combustion will take place and not all the carbon is fully oxidised
- Some carbon is only partially oxidised to form carbon monoxide
- For example, the incomplete combustion of octane to form carbon monoxide
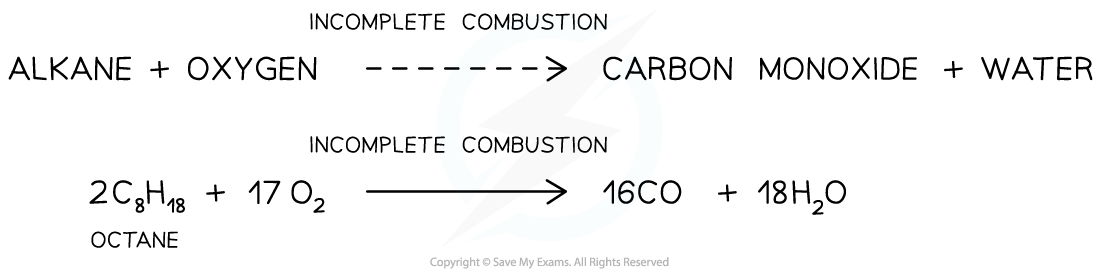
The incomplete combustion of alkanes
- Carbon monoxide is a toxic gas as it will bind to haemoglobin in blood which can then no longer bind oxygen
- As no oxygen can be transported around the body, victims will feel dizzy, lose consciousness and if not removed from the carbon monoxide, they can die
- Carbon monoxide is extra dangerous as it is odourless (it doesn’t smell) and will not be noticed
- Incomplete combustion often takes place inside a car engine due to a limited amount of oxygen present
Free-radical substitution of alkanes
- Alkanes can undergo free-radical substitution in which a hydrogen atom gets substituted by a halogen (chlorine/bromine)
- Since alkanes are very unreactive, ultraviolet light (sunlight) is needed for this substitution reaction to occur
- The free-radical substitution reaction consists of three steps:
- In the initiation step, the halogen bond (Cl-Cl or Br-Br) is broken by UV energy to form two radicals
- These radicals create further radicals in a chain type reaction called the propagation step
- The reaction is terminated when two radicals collide with each other in a termination step
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









