- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记3.1.11 Stereoisomerism
Stereoisomerism: Geometrical & Optical
- Stereoisomers are compounds that have the same atoms connected to each other, however the atoms are differently arranged in space
- There are two types of stereoisomerism:
- Geometrical (cis/trans) isomerism
- Optical isomerism
Geometrical (cis/trans) isomerism
- Geometrical isomerism is seen in unsaturated (double bond containing) or ring compounds that have the same molecular formula and order of atoms (the atoms are connected similarly to each other) but different shapes
- Cis/trans nomenclature is used to distinguish between the isomers
- Cis isomers have functional groups on the same side of the double bond/carbon ring
- Trans isomers have functional groups on opposite sides of the double bond/carbon ring
 Geometrical isomerism in unsaturated compounds
Geometrical isomerism in unsaturated compounds
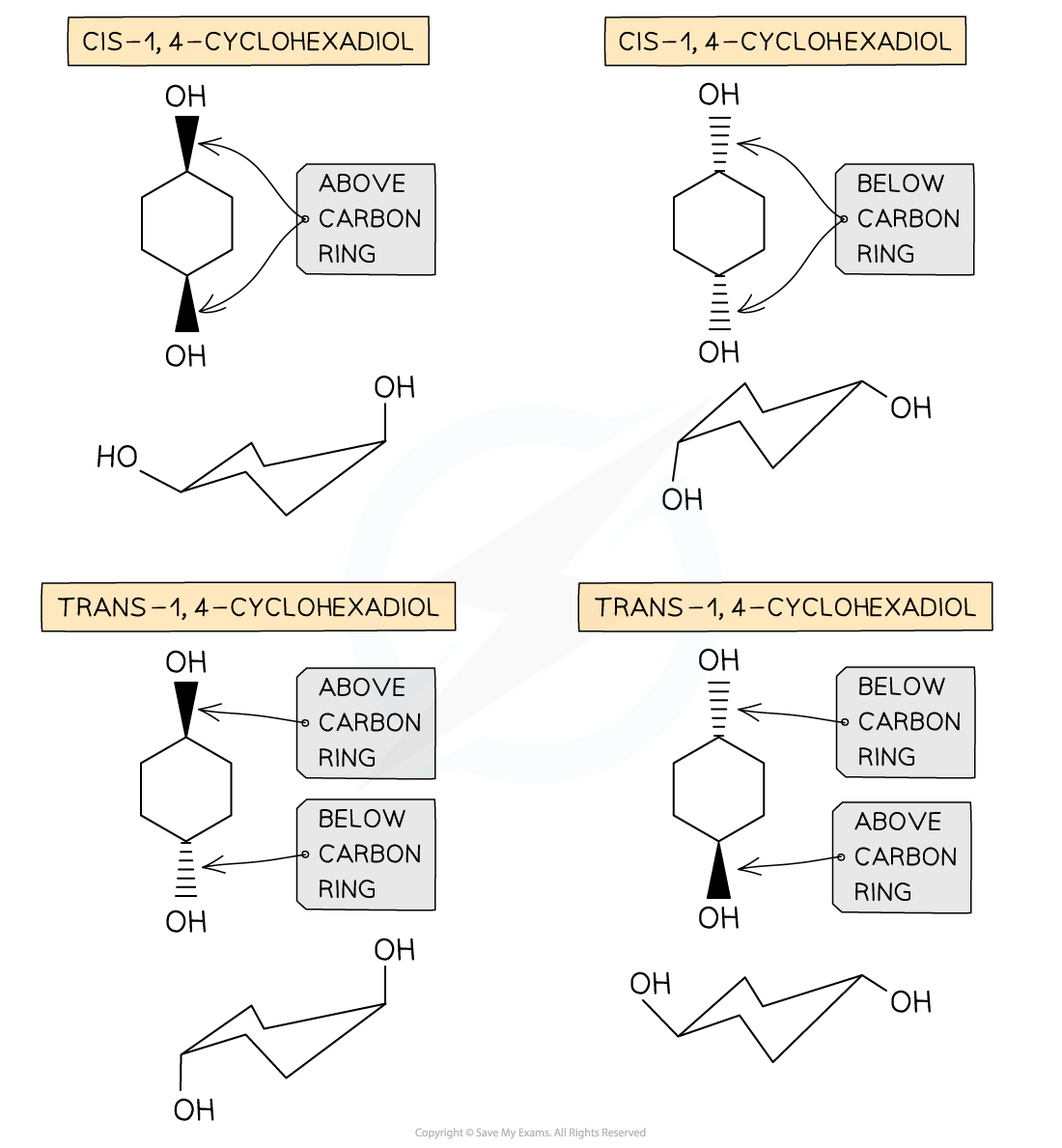
Geometrical isomerism in cyclic compounds
- This causes the compounds to have different chemical and physical properties
- For example, they may have different reaction rates for the same reaction (chemical property) or different melting/boiling points (physical property)
Optical isomerism
- Optical isomers arise when a carbon atom in a molecule is bonded to four different atoms or groups of atoms
- The carbon atom is ‘asymmetric’ as there is no plane of symmetric in the molecule and is also called the chiral centre of the molecule
- The two different optical isomers are also called enantiomers
- Just like the left hand cannot be superimposed on the right hand, enantiomers too are non-superimposable
- Enantiomers are mirror images of each other
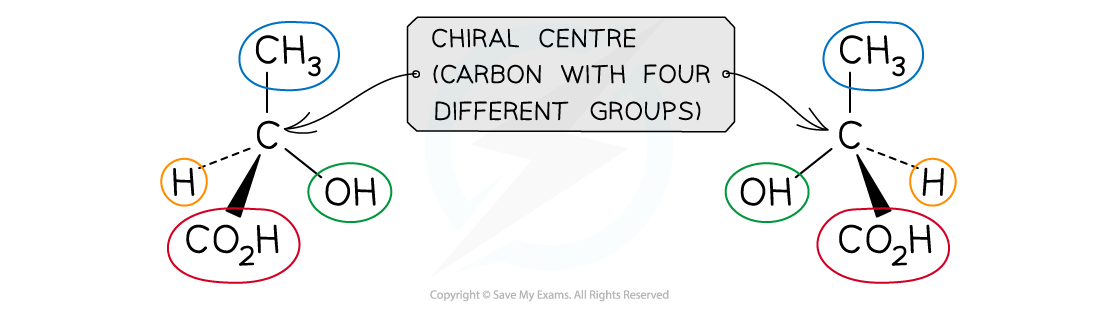
Both molecules are made up of the same atoms which are bonded to each other identically, however the chiral centre (carbon with four different groups) gives rise to optical isomerism
- Optical isomers differ in their ability to rotate the plane of polarised light
- One enantiomer will rotate it clockwise and the other anticlockwise
A2 only
- Normal light is unpolarised and consists of electric and magnetic fields that vibrate at right angles to each other in every possible direction
- When the unpolarised light passes through a polariser, the light gets polarised causing it to vibrate in only one plane
- A pair of optical isomers will rotate the plane of polarised light by equal amounts in opposite direction
- When equal amounts of the enantiomers are present in solution, the plane of polarised light doesn’t change
- As the enantiomers cancel out each other’s effect
- A solution with equal amounts of both enantiomers is also called a racemic mixture
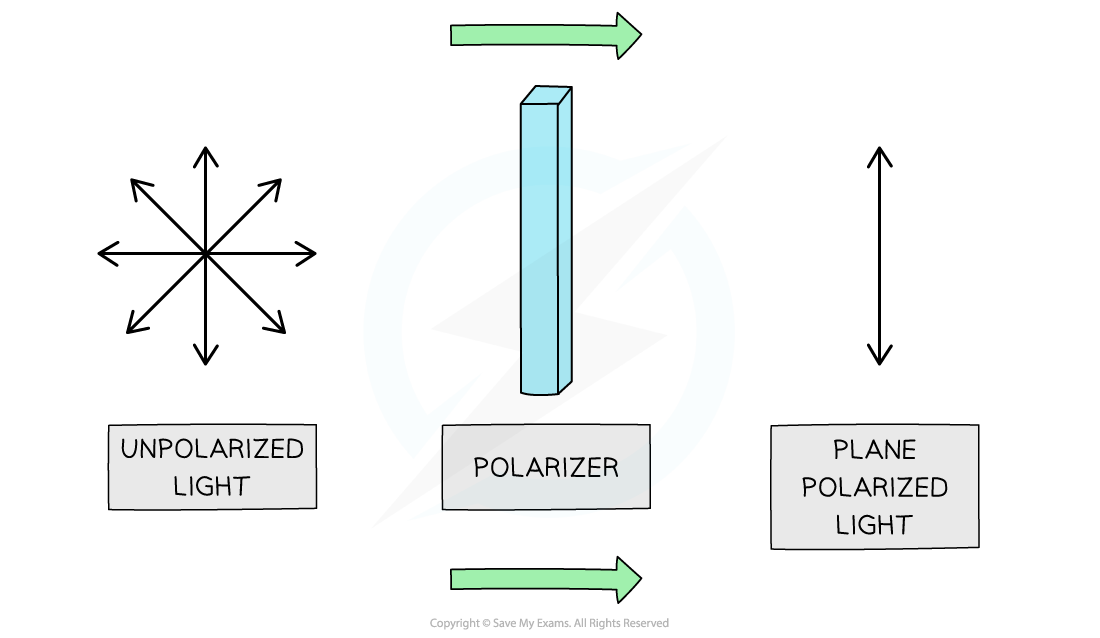 Light consists of vibrations in all possible directions however when it passes through a polariser the light gets polarised and vibrates in only one plane: enantiomers cause the plane of polarised light to rotate clockwise or anticlockwise
Light consists of vibrations in all possible directions however when it passes through a polariser the light gets polarised and vibrates in only one plane: enantiomers cause the plane of polarised light to rotate clockwise or anticlockwise
Geometrical Isomerism in Alkenes
Unsaturated compounds
- In unsaturated compounds, the groups attached to the C=C carbons remain fixed in their position
- This is because free rotation of the bonds about the C=C bond is not possible due to the presence of a π bond
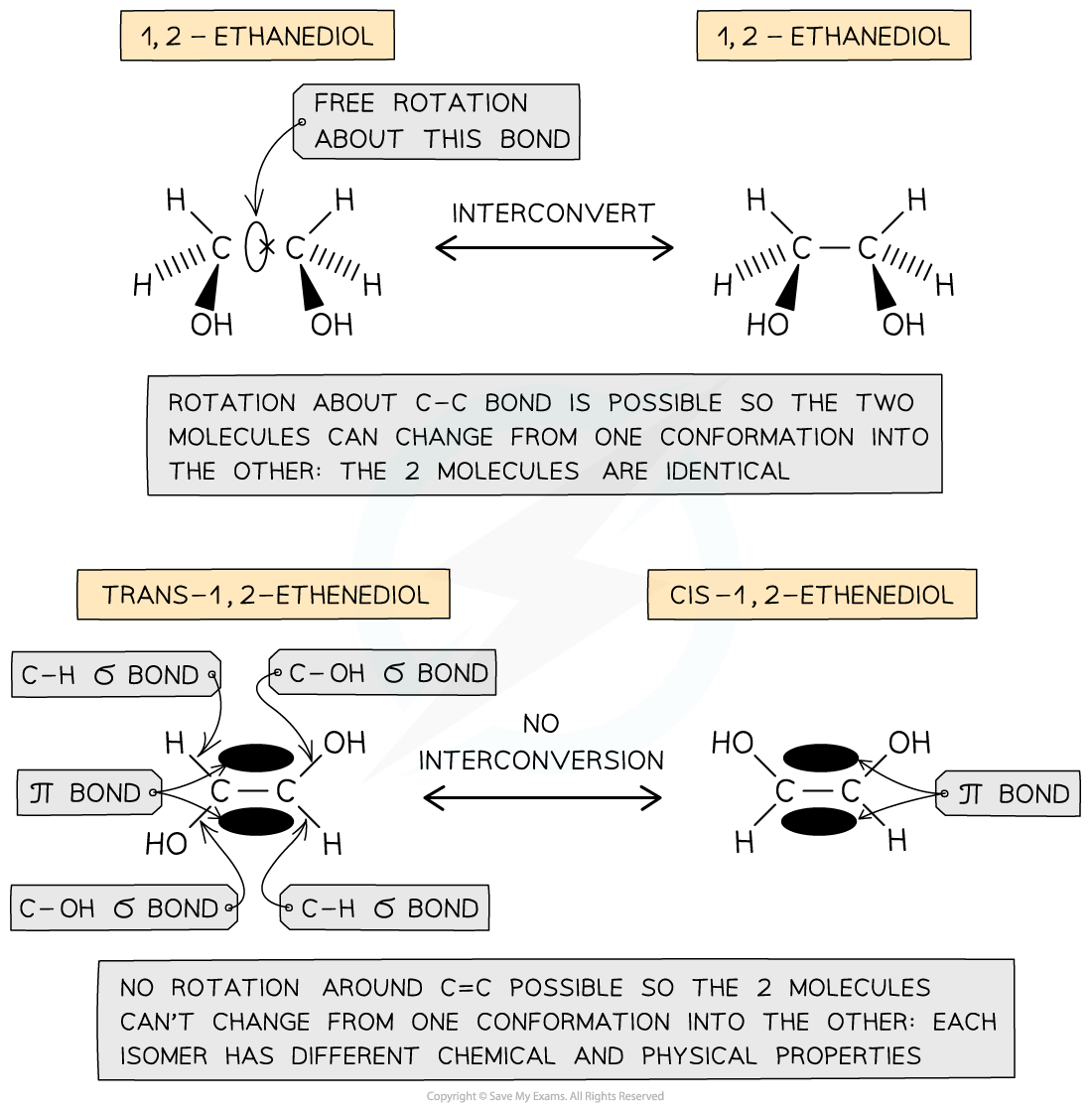
The presence of a π bond in unsaturated compounds restricts rotation about the C=C bond forcing the groups to remain fixed in their position and giving rise to the formation of geometrical isomers
Exam Tip
Geometrical isomerism is also possible in cyclic compounds because there is limited rotation about C-C single bonds that make up the rings.Therefore, the substitutions in cyclic compounds are fixed in their position (to stay either above or below the ring of carbon atoms).
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









