- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记2.4.1 Nitrogen and its Compounds
Reactivity of Nitrogen
- Nitrogen is a diatomic molecule and the main unreactive gas in air
- 78% of air is nitrogen gas
- The lack of reactivity of nitrogen gas can be explained by looking at its intramolecular bonds
- Intramolecular bonds are the bonds within a molecule
Bonding in nitrogen
- The electron configuration of a nitrogen atom is 1s2 2s2 2p3
- To achieve a full outer shell of electrons, it needs to gain three electrons
- Nitrogen atoms therefore form a triple covalent bond between two nitrogen atoms in which they share three electrons with each other
 The diagram shows a triple covalent bond between two nitrogen atoms to achieve a full outer shell of electrons
The diagram shows a triple covalent bond between two nitrogen atoms to achieve a full outer shell of electrons
- The bond enthalpy of the nitrogen triple bond is 1000 kJ mol-1
- This means that 1000 kJ of energy is needed to break one mole of N2 triple bond
- As it is so difficult to break the nitrogen triple bond, nitrogen and oxygen gas in air will not react with each other
- Only under extreme conditions will nitrogen gas react (eg. during a thunderstorm)
Polarity of nitrogen
- The electrons in a nitrogen molecule are shared equally between the two nitrogen atoms
- Therefore, nitrogen molecules are nonpolar molecules
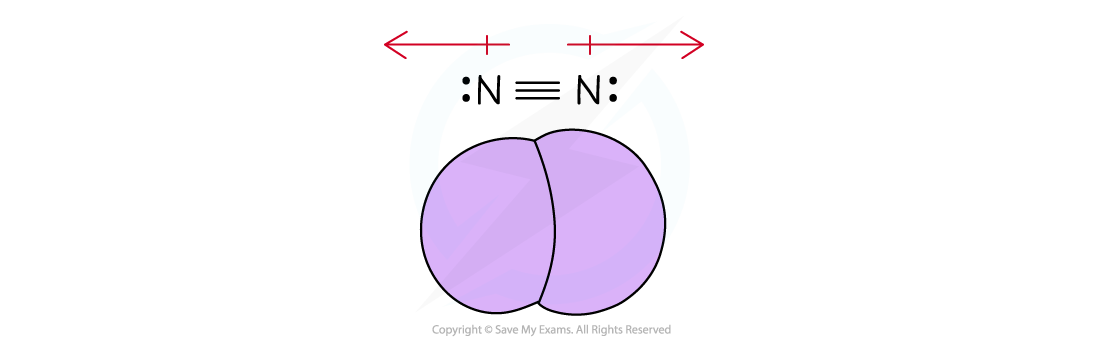
Since the electronegativity of the two nitrogen atoms is the same, the will pull the electrons towards them equally so overall the molecule is nonpolar
- Due to the lack of polarity, nitrogen gas is not attracted to or likely to react with other molecules the way polar molecules would
Exam Tip
Nitrogen is very unreactive due to the lack of polarity and strength of its triple bond.
Properties of Ammonia
- Ammonia is a compound of nitrogen and will turn damp red litmus paper blue as it is an alkaline gas
- Ammonia is made on a large scale in industry using the Haber process:
N2(g) + 3H2(g) ⇌ 2NH3(g)
Basicity of ammonia
- Ammonia can act as a Brønsted–Lowry base by accepting a proton (H+) using the lone pair of electrons on the nitrogen atom to form an ammonium ion:
NH3(aq) + H+(aq) → NH4+(aq)
- In an aqueous solution of ammonia, an equilibrium mixture is established
NH3(aq) + H2O(l) ⇌ NH4+(aq) + OH-(aq)
- Since the position of the equilibrium lies well over to the left the ammonia solution is only weakly alkaline
- There is a higher concentration of ammonia molecules than hydroxide ions in solution
- Ammonia is therefore a weak base
Structure & formation of ammonium ion
- The ammonium ion is formed by an acid-base reaction of ammonia with water:
NH3(aq) + H2O(l) ⇌ NH4+(aq) + OH-(aq)
- The nitrogen in ammonia is covalently bonded to three hydrogen atoms and has one lone pair of electrons causing the ammonia molecule to have a pyramidal shape
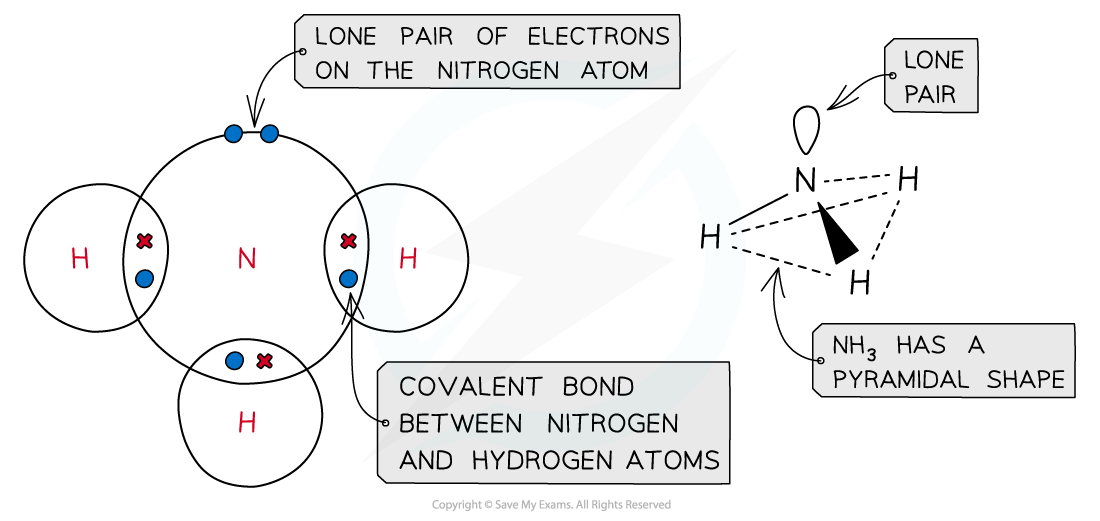
Ammonia has a pyramidal shape due to its lone pair of electrons
- The nitrogen atom in ammonia uses its lone pair of electrons to form a dative bond with a proton to form the ammonium ion
- The ammonium ion has a tetrahedral shape in which all bonds have the same length
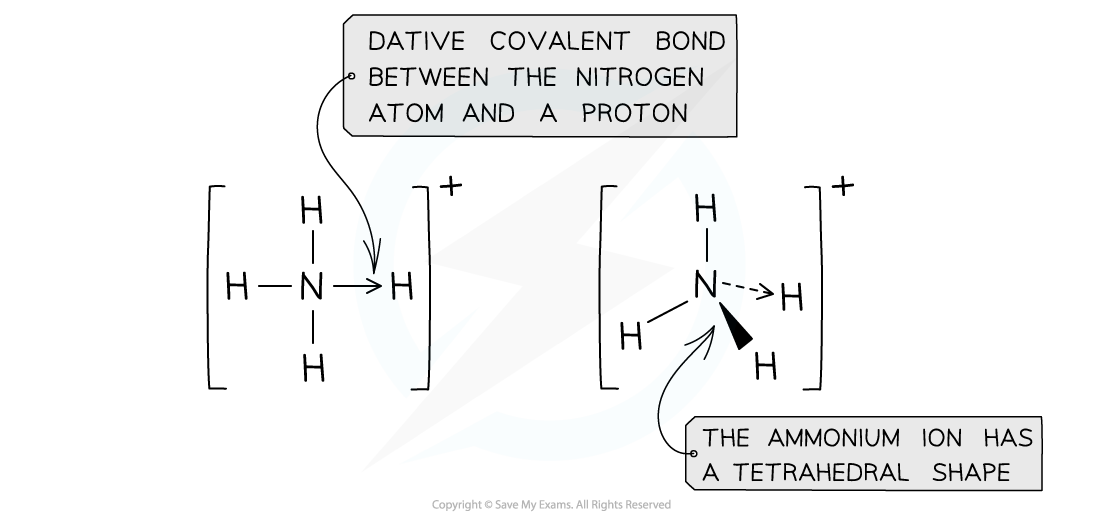 The tetrahedral shape of the ammonium ion
The tetrahedral shape of the ammonium ion
Preparation of ammonia gas from an ammonium salt
- Ammonia gas can be prepared from an ammonium salt and a base in an acid-base reaction:
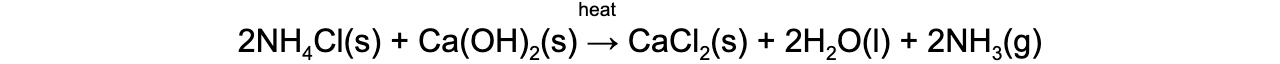
- Ammonium chloride (NH4Cl) and calcium hydroxide (Ca(OH)2) are mixed together and then heated
- NH4+acts as an acid (proton donor) and OH- acts as a base (proton acceptor)
- This acid-base reaction can be used to test if an unknown solution contains ammonium ions
- If the unknown solution does contain ammonium ions, it will react with calcium hydroxide to form ammonia gas
- This ammonia gas will turn damp red litmus paper blue
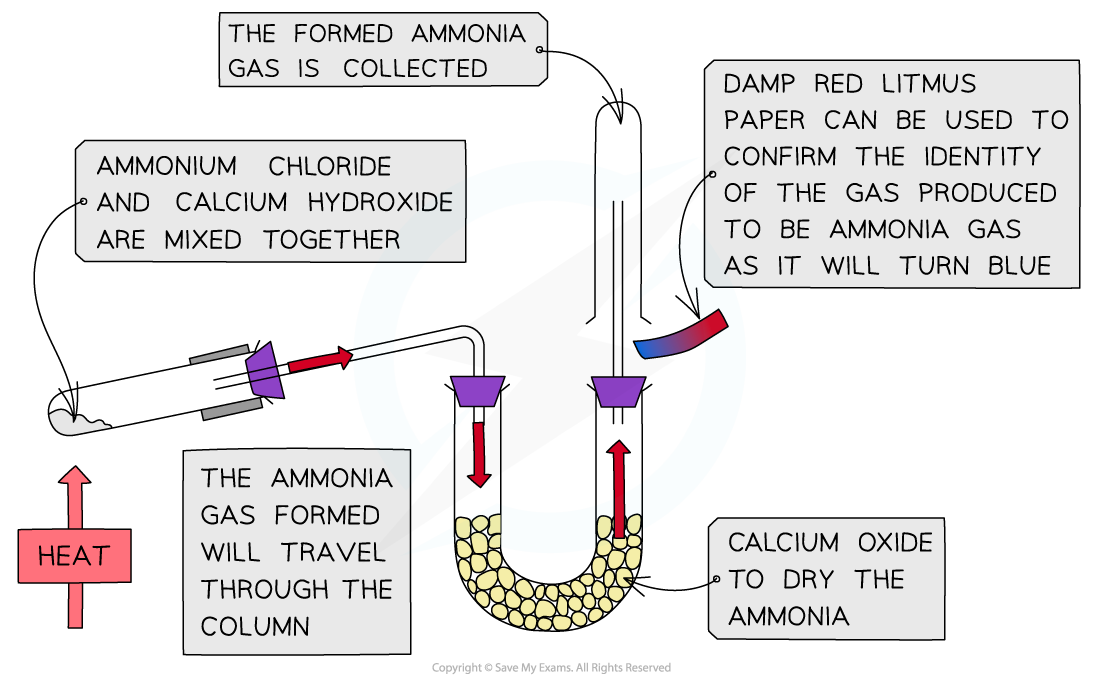
The diagram shows the apparatus set up for the preparation of ammonia gas from an ammonium salt and calcium hydroxide
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








