- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记2.2.5 Group 2: Trends in Solubility of Hydroxides & Sulfates
Trends in Solubility in Group 2 Hydroxides & Sulfates
Group 2 hydroxides
- Going down the group, the solutions formed from the reaction of Group 2 oxides with water become more alkaline
- When the oxides are dissolved in water, the following ionic reaction takes place:
O2- (aq) + H2O(l) → 2OH- (aq)
- The higher the concentration of OH- ions formed, the more alkaline the solution
- The alkalinity of the formed solution can therefore be explained by the solubility of the Group 2 hydroxides
Solubility of the Group 2 hydroxides table
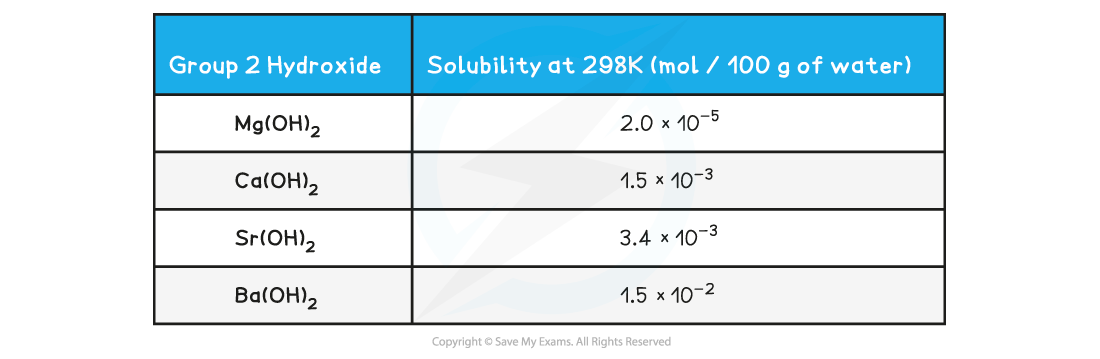
- The hydroxides dissolve in water as follows:
X(OH)2 (aq) → X(aq) + 2OH- (aq)
Where X is the Group 2 element
- When the metal oxides react with water, a Group 2 hydroxide is formed
- Going down the group, the solubility of these hydroxides increases
- This means that the concentration of OH- ions increases, increasing the pH of the solution
- As a result, going down the group, the alkalinity of the solution formed increases when Group 2 oxides react with water
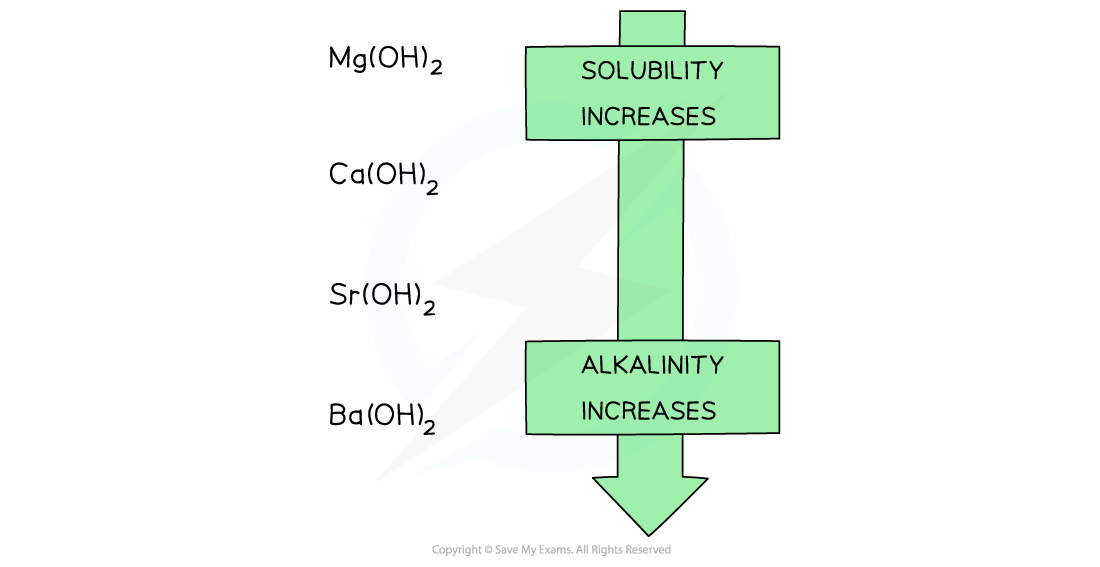
Going down the group, the solubility of the hydroxides increases which means that the solutions formed from the reactions of the Group 2 metal oxides and water become more alkaline going down the group
Group 2 sulfates
- The solubility of the Group 2 sulfates decreasing going down the group
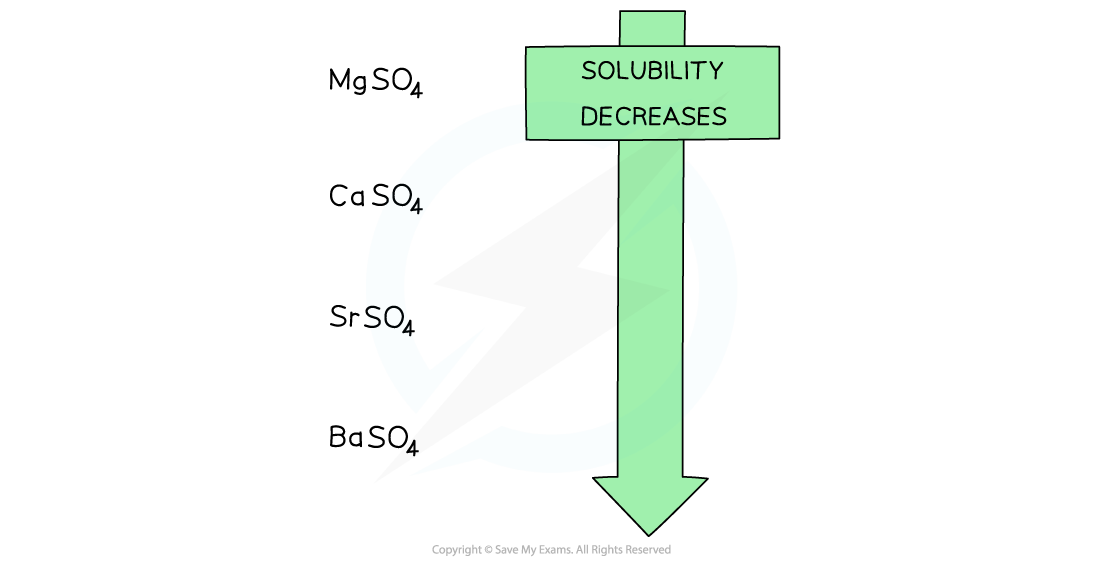
Going down the group, the solubility of the sulfates decreases
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









