- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记2.2.1 Reactions of Group 2 Elements
Reactions of Group 2 Elements
- The Group 2 elements react with oxygen, water and dilute acids
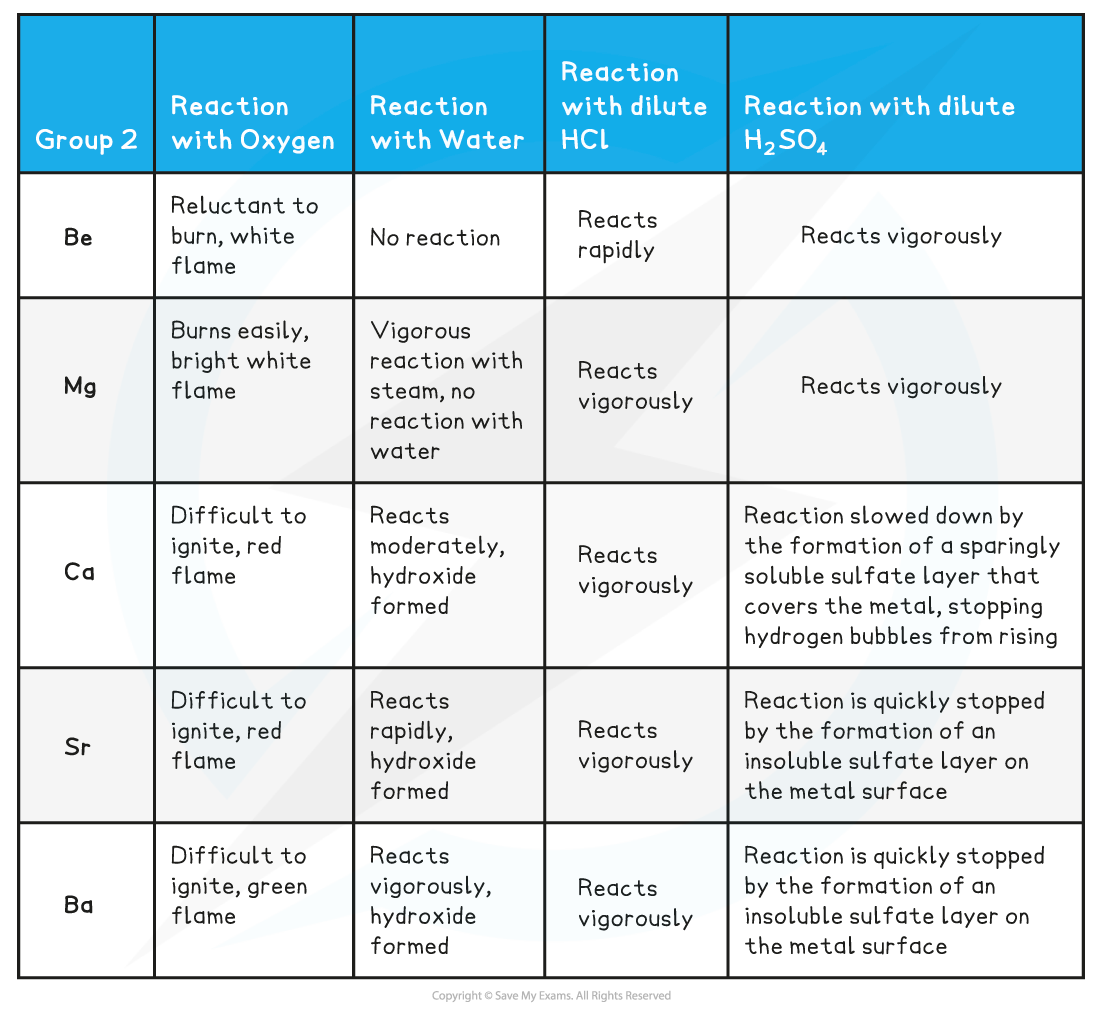
Group 2 reactions table
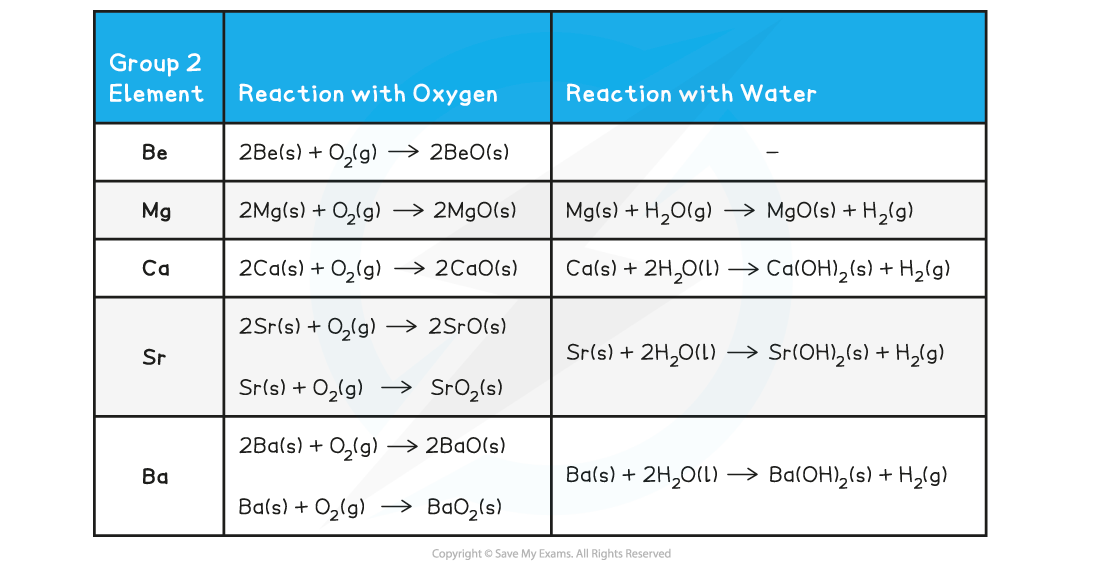
Group 2 reactions with oxygen & water chemical equations
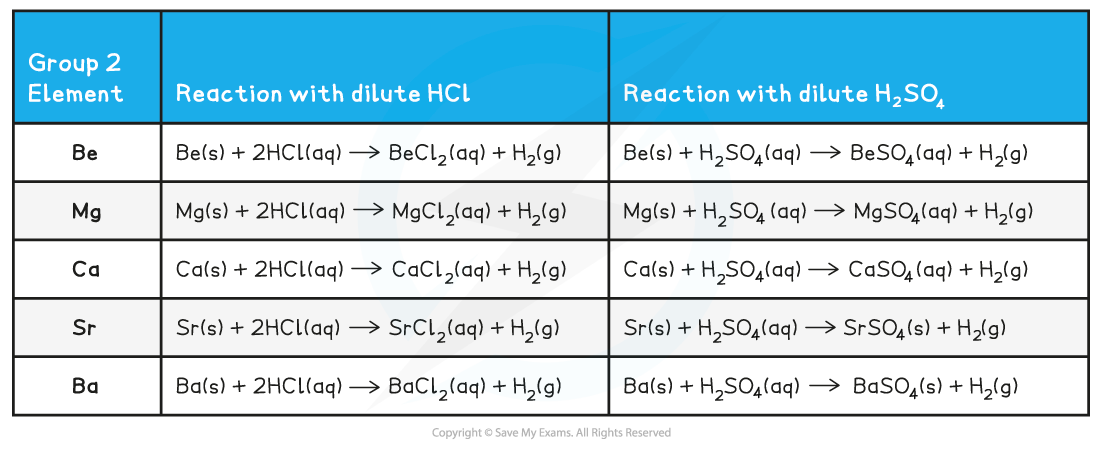
Group 2 reactions with dilute hydrochloric acid & dilute sulfuric acid chemical equations
- The reaction of all metals with oxygen follows the following general equation:
2M(s) + O2(g) → 2MO(s)
Where M is any metal in Group 2
Remember than Sr and Ba also form MO2
- The reaction of all metals with water follows the following general equation:
M(s) + 2H2O(l) → M(OH)2(s) + H2(g)
Except for, Be which does not react with water
- The reaction of all metals with dilute HCl follows the following general equation:
M(s) + 2HCl(aq) → MCl2(aq) + H2(g)
- The reaction of all metals with dilute H2SO4 follows the following general equation:
M(s) + H2SO4(aq) → MSO4(aq) + H2(g)
Remember that SrSO4 and BaSO4 are insoluble
Exam Tip
Learn the general equation for the reaction with oxygen, water and dilute HCl/H2SO4 and the exceptions instead of memorizing the entire table!
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









