- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记2.1.7 Period 3 Chlorides & Oxides
Bonding in Period 3 Chlorides & Oxides
Period 3 chlorides
- The bonding and structure of the Period 3 elements are summarised in the table below:
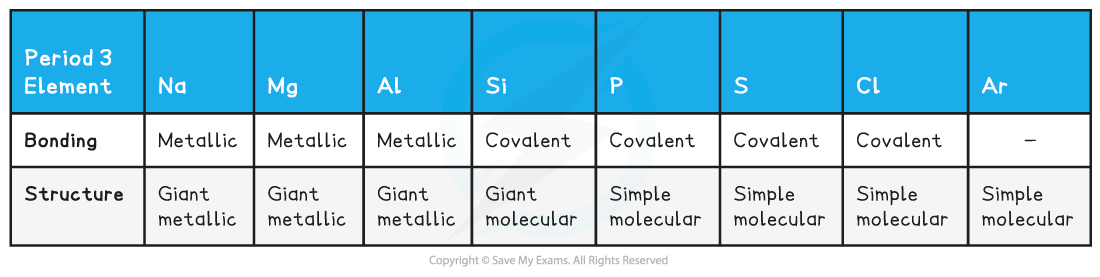
- The table shows that Na, Mg and Al are metallic elements which form positive ions arranged in a giant lattice in which the ions are held together by a ‘sea’ of delocalised electrons around them
- The electrons in the ‘sea’ of delocalised electrons are those from the valence shell of the atoms
- Na will donate one electron into the ‘sea’ of delocalised electrons, Mg will donate two and Al three electrons
- As a result of this, the metallic bonding in Al is stronger than in Na
- This is because the electrostatic forces between a 3+ ion and the larger number of negatively charged delocalised electrons is much larger compared to a 1+ ion and the smaller number of delocalised electrons in Na
- Because of this, the melting points increase going from Na to Al
- Si has the highest melting point due to its giant molecular structure in which each Si atom is held to its neighbouring Si atoms by strong covalent bonds
- P, S, Cl and Ar are non-metallic elements and exist as simple molecules (P4, S8, Cl2 and Ar as single atom)
- The covalent bonds within the molecules are strong, however between the molecules there are only weak instantaneous dipole-induced dipole forces
- It doesn’t take much energy to break these intermolecular forces
- Therefore, the melting points decrease going from P to Ar (note that the melting point of S is higher than that of P as sulphur exists as larger S8 molecules compared to the smaller P4 molecule)
- The presence of a ‘sea’ of delocalised electrons also determines whether the element is a good conductor or not
- Going across the period the electrical conductivity of the elements decreases due to lack of delocalised electrons
- The electronegativities of the Period 3 elements therefore determines the chemical bonding and structure of their chlorides and oxides
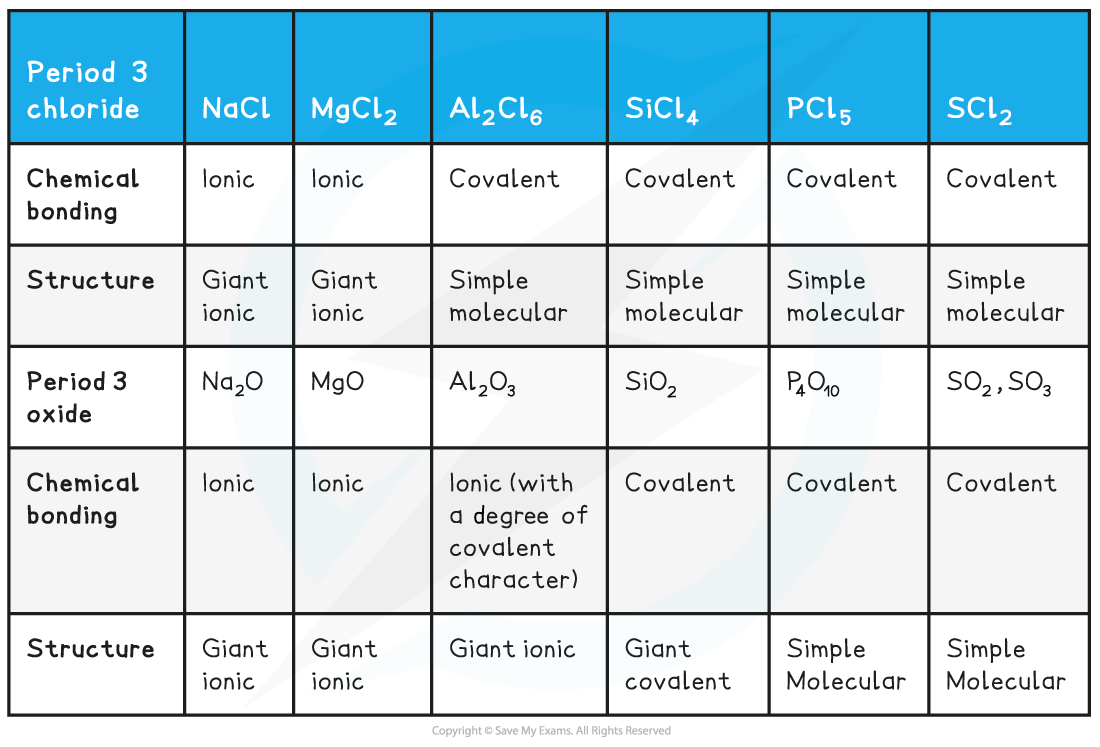
- Going across Period 3, their chlorides and oxidised become more covalent and their structure shifts from a giant ionic to a simple molecular structure
- Their reactions with water become more vigorous as a result of this as it becomes easier to hydrolyse the chlorides and oxides
转载自savemyexams

在线登记
最新发布
翰林课程体验,退费流程快速投诉邮箱: yuxi@linstitute.net 沪ICP备2023009024号-1








