- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记2.1.4 Period 3 Oxides & Hydroxides: Acid/Base Behaviour
Acid / Base Behaviour of Period 3 Oxides & Hydroxides
Period 3 oxides
- Aluminium oxide is amphoteric which means that it can act both as a base (and react with an acid such as HCl) and an acid (and react with a base such as NaOH)
Acidic & basic nature of the Period 3 oxides
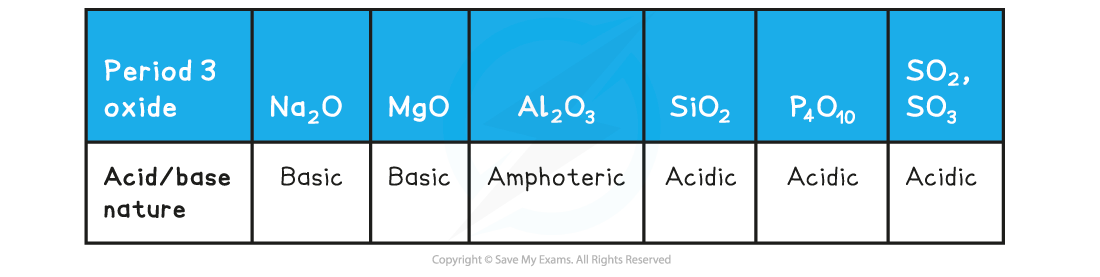
Reactions of the Period 3 oxides with acid/base table
- The acidic and basic nature of the Period 3 elements can be explained by looking at their structure, bonding and the Period 3 elements’ electronegativity
Structure, bonding & electronegativity of the Period 3 elements table
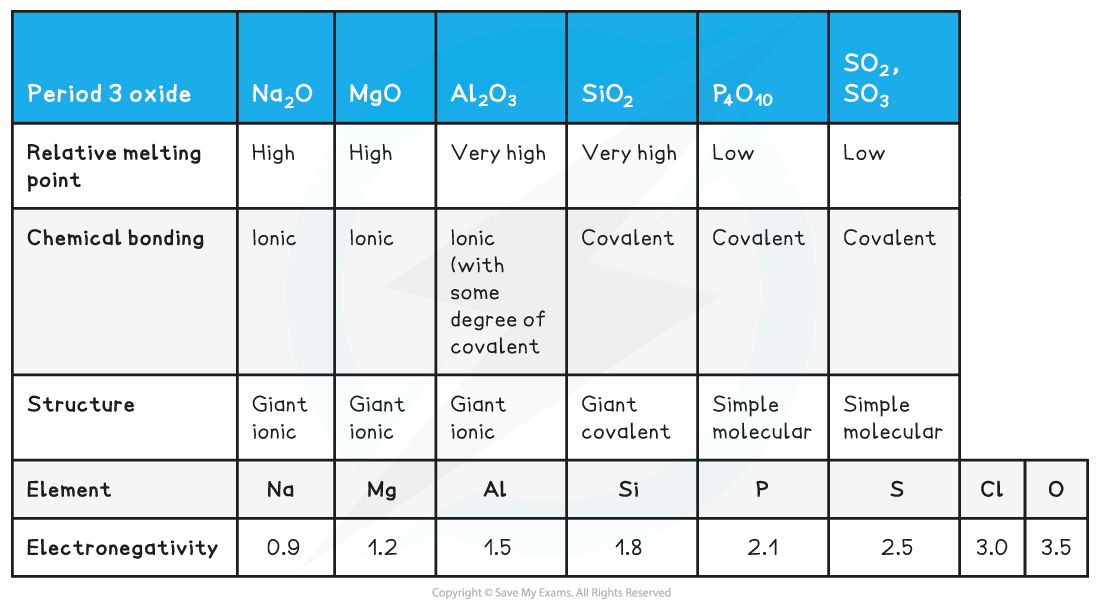
- The difference in electronegativity between oxygen and Na, Mg and Al is the largest
- Electrons will therefore be transferred to oxygen when forming oxides giving the oxide an ionic binding
- The oxides of Si, P and S will share the electrons with the oxygen to form covalently bonded oxides
- The giant ionic and giant covalent structured oxides will have high melting points as it is difficult to break the structures apart
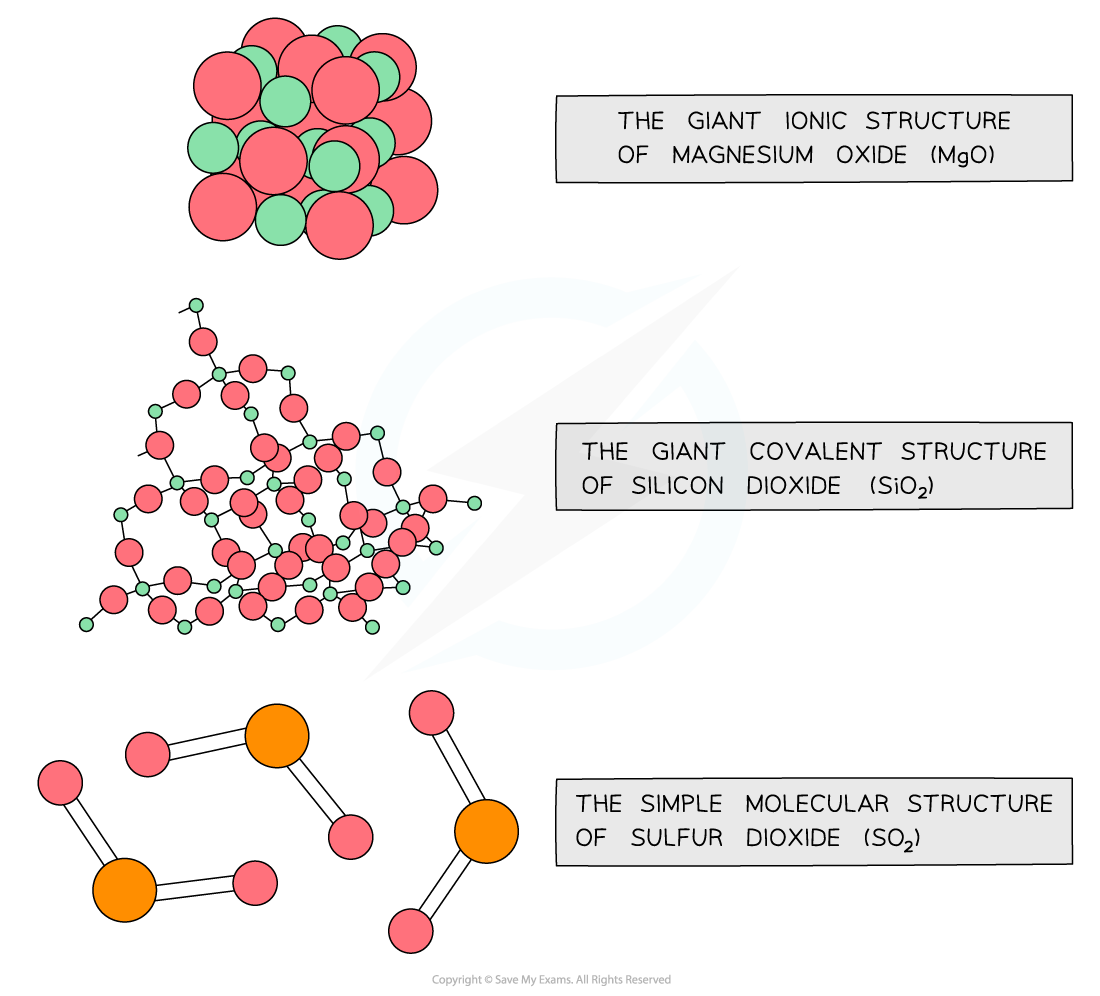 The diagram shows the structure of some Period 3 oxides
The diagram shows the structure of some Period 3 oxides
- The oxides of Na and Mg which show purely ionic bonding produce alkaline solutions with water as their oxide ions (O2-) become hydroxide ions (OH-):
O2-(aq) + H2O(l) → 2OH-(aq)
- The oxides of P and S which show purely covalent bonding produce acidic solutions with water because when these oxides react with water, they form an acid which donates H+ ions to water
- Eg. SO3 reacts with water as follows:
SO3(g) + H2O(l) → H2SO4(aq)
-
- The H2SO4 is an acid which will donate a H+ to water:
H2SO4(aq) + H2O(l) → H3O+ (aq) + HSO4-(aq)
- Al and Si are insoluble and when they react with hot, concentrated alkaline solution they act as a base and form a salt
- This behaviour is very typical of a covalently bonded oxide
- Al can also react with acidic solutions to form a salt and water
- This behaviour is very typical of an ionic bonded metal oxide
- This behaviour of Al proves that the chemical bonding in aluminium oxide is not purely ionic nor covalent: it is amphoteric
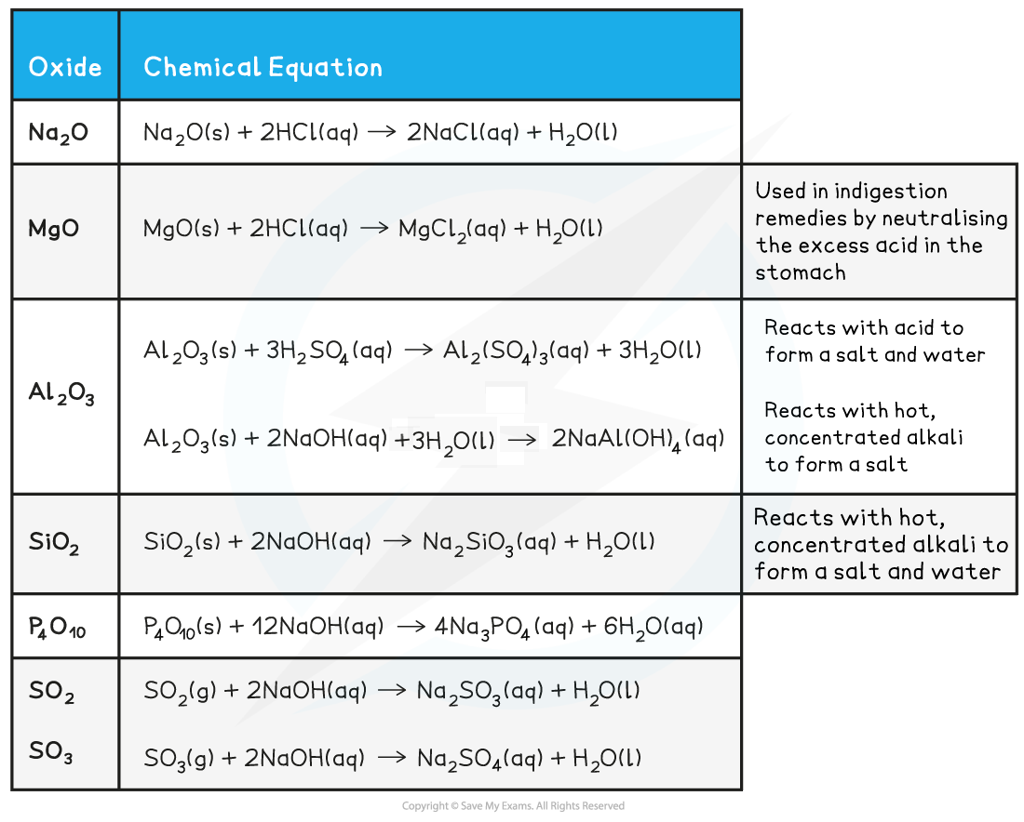
Period 3 hydroxide
- NaOH is a strong base and will react with acids to form a salt and water:
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
- Mg(OH)2 is also a basic compound which is often used in indigestion remedies by neutralising the excess acid in the stomach to relieve pain:
Mg(OH)2(s) + 2HCl(aq) → MgCl2(aq) + 2H2O(l)
- Al(OH)3 is amphoteric and can acts both as an acid and base:
Al(OH)3(s) + 3HCl(aq) → AlCl3(s) + 3H2O(l)
Al(OH)3(s) + NaOH(aq) → NaAl(OH)4(aq)
Exam Tip
Electronegativity is the power of an element to draw the electrons towards itself in a covalent bond.For example, in Na2O the oxygen will draw the electrons more strongly towards itself than sodium does.
转载自savemyexams


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








