- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记2.1.1 Period 3 Elements: Physical Properties
Properties of the Elements in Period 3
- Elements in the periodic table are arranged in order of increasing atomic number and placed in vertical columns (groups) and horizontal rows (periods)
- The elements across the periods show repeating patterns in chemical and physical properties
- This is called periodicity
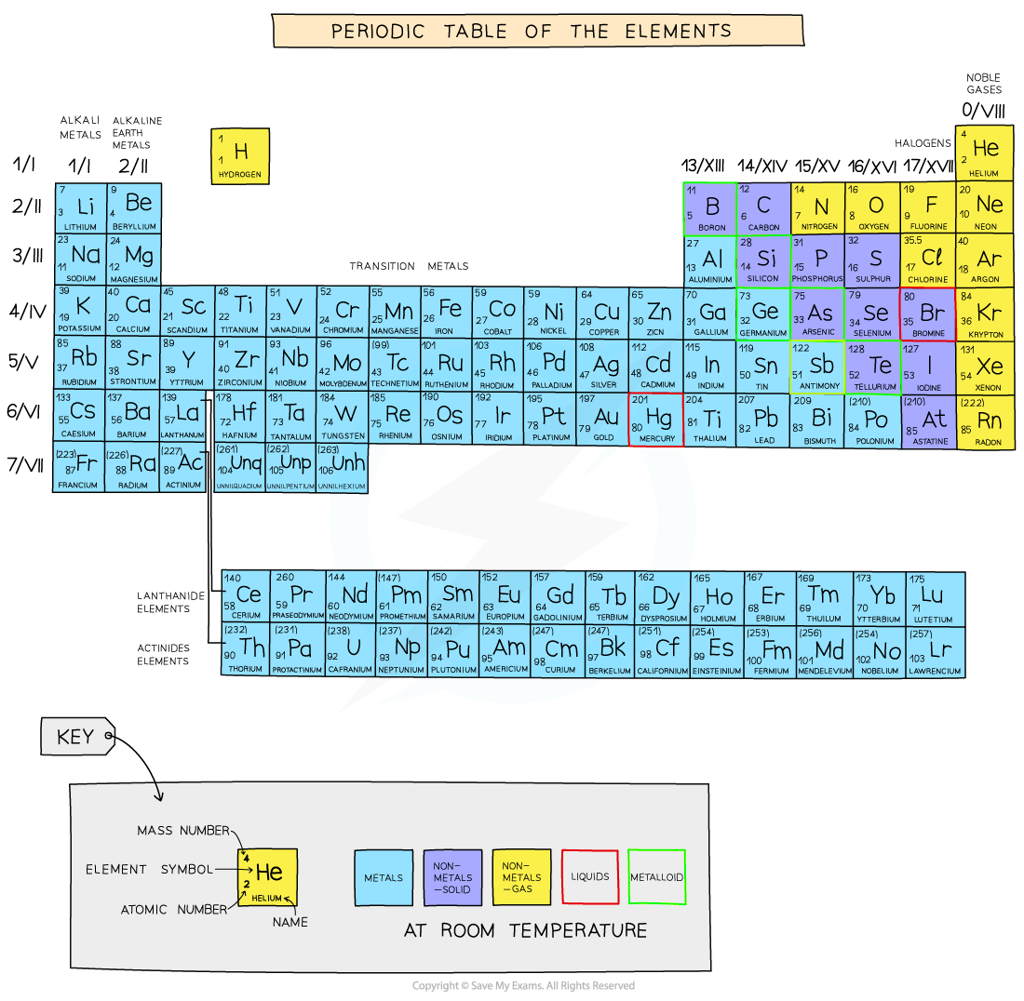
All elements are arranged in the order of increasing atomic number from left to right
Atomic radius
- The atomic radius is the distance between the nucleus and the outermost electron of an atom
- The atomic radius is measured by taking two atoms of the same element, measuring the distance between their nuclei and then halving this distance
- In metals this is also called the metallic radius and in non-metals, the covalent radius
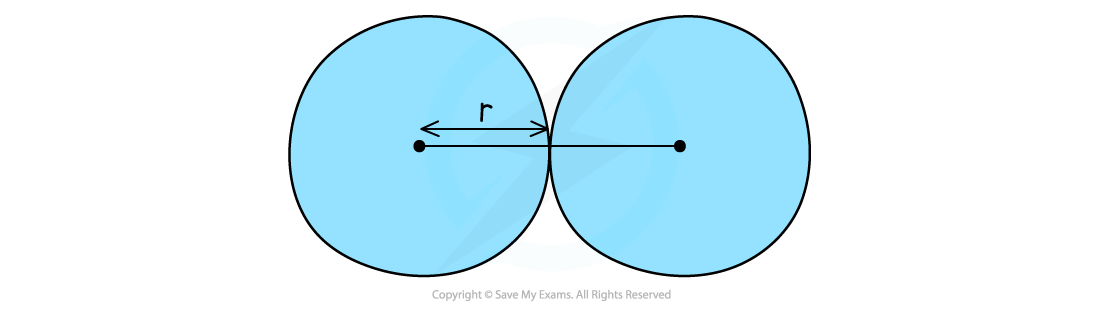 The atomic radius gives a measure of the size of atoms
The atomic radius gives a measure of the size of atoms
Atomic radii of Period 3 elements table
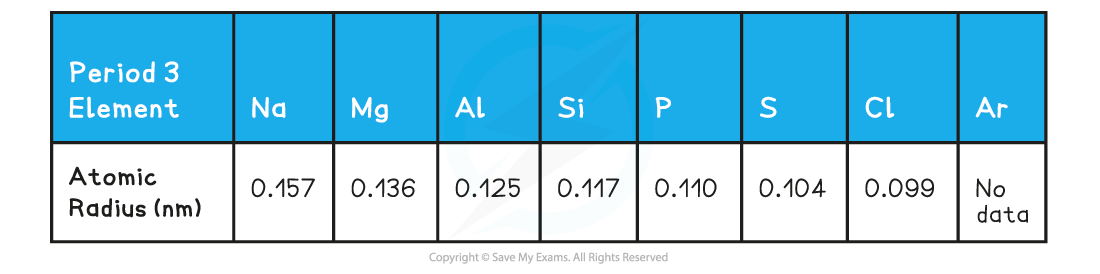
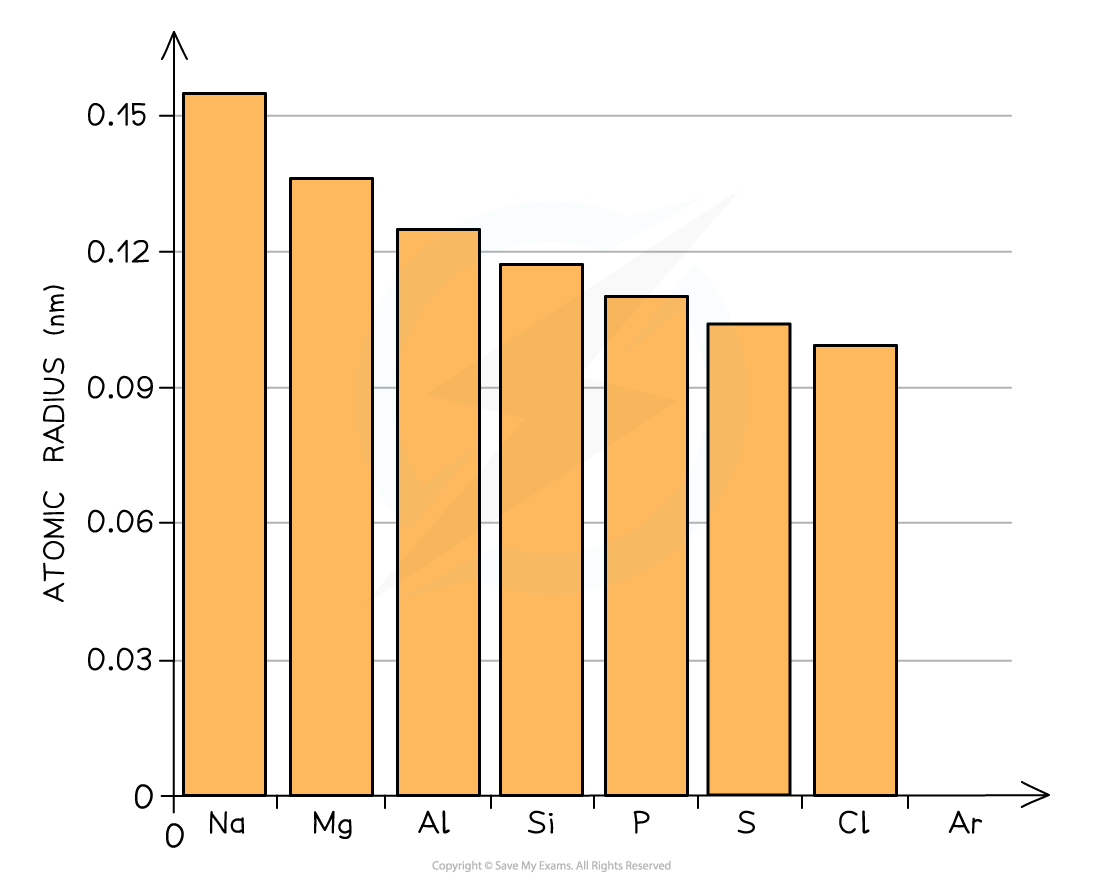
The graph shows a decrease in atomic radii of Period 3 elements across the period
- Across the period, the atomic radii decrease
- This is because the number of protons (the nuclear charge) and the number of electrons increases by one every time you go an element to the right
- The elements in a period all have the same number of shells (so the shielding effect is the same)
- This means that as you go across the period the nucleus attracts the electrons more strongly pulling them closer to the nucleus
- Because of this, the atomic radius (and thus the size of the atoms) decreases across the period
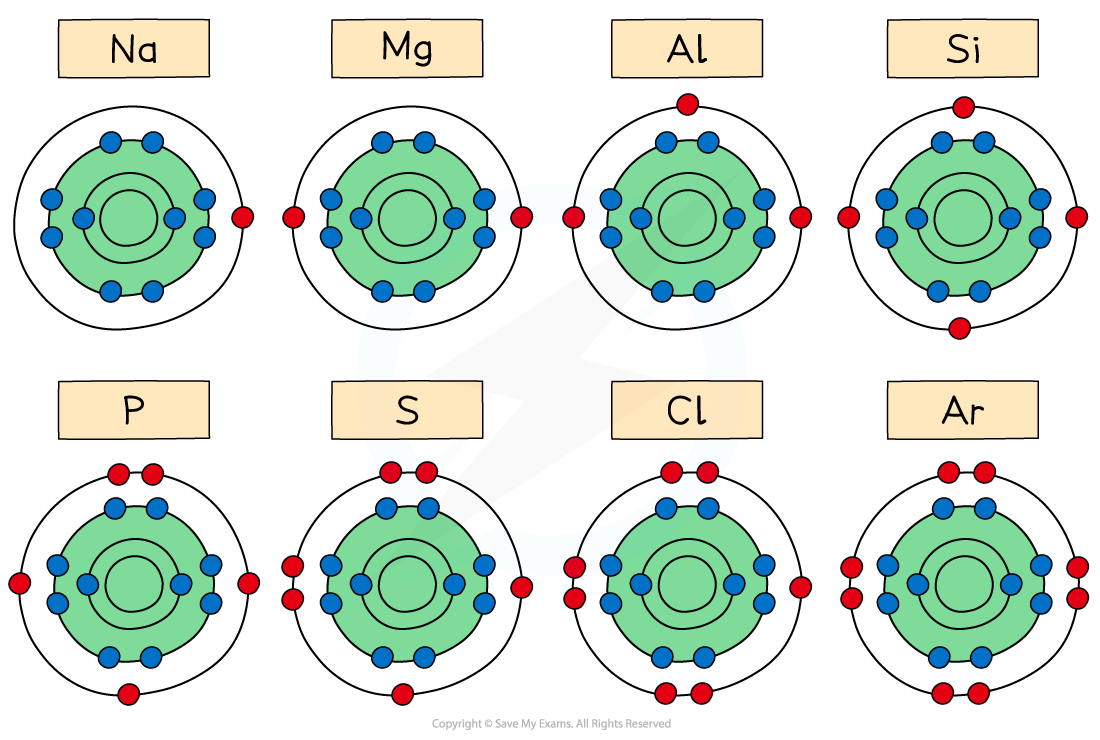
The diagram shows that across Period 3, the elements gain extra electrons in the same principal quantum shell
Ionic radius
- The ionic radius is the distance between the nucleus and the outermost electron of an ion
- Metals produce positively charged ions (cations) whereas nonmetals produce negatively charged ions (anions)
- The cations have lost their valence electrons which causes them to be much smaller than their parent atoms
- Because there are less electrons, this also means that there is less shielding of the outer electrons
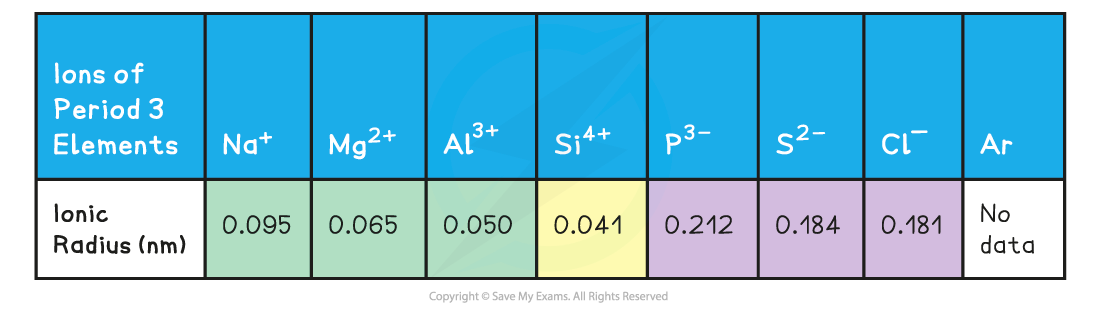
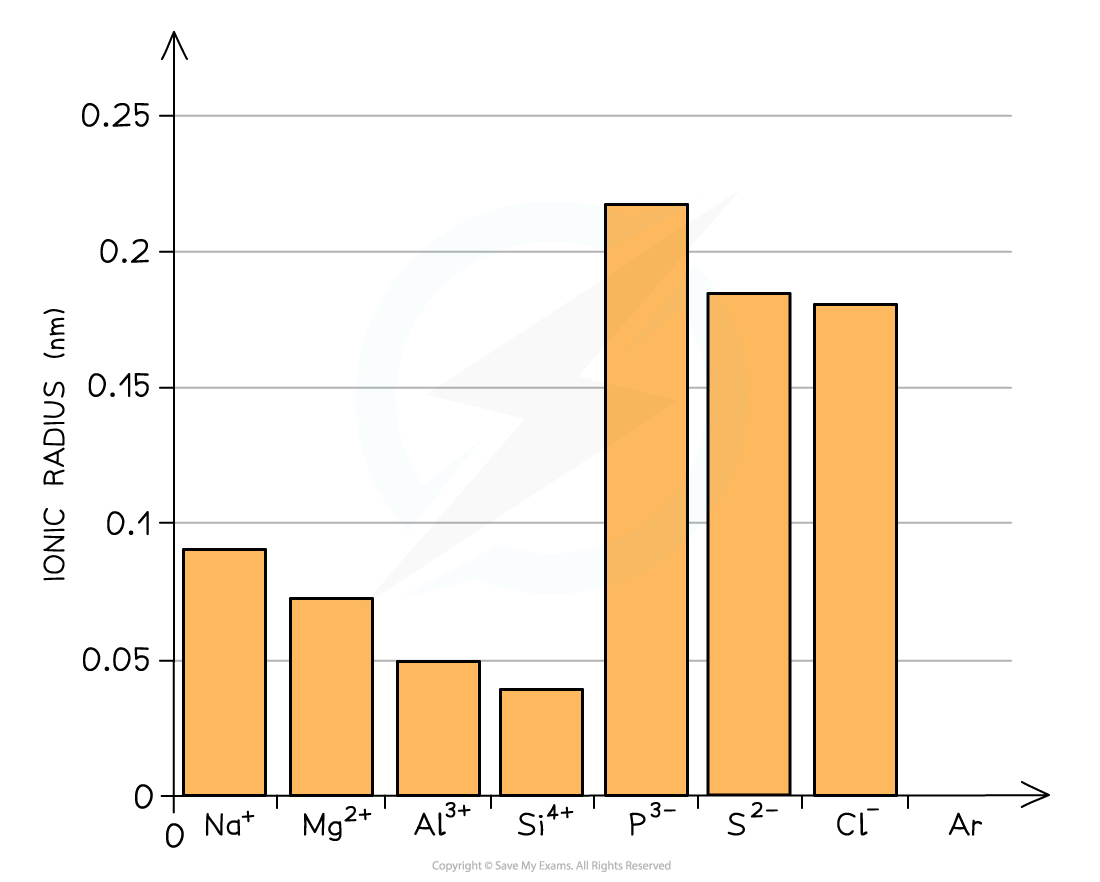
- Going across the period from Na+ to Si4+ the ions get smaller due to the increasing nuclear charge attracting the outer electrons in the second principal quantum shell nucleus (which has an increasing atomic number)
- The anions are larger than their original parent atoms because each atom has gained one or more electrons in their third principal quantum shell
- This increases the repulsion between electrons, while the nuclear charge is still the same, causing the electron cloud to spread out
- Going across P3- to Cl- the ionic radii decreases as the nuclear charge increases across the period and fewer electrons are gained by the atoms (P gains 3 electrons, S 2 electrons and Cl 1 electron)
Ionic radii of ions of Period 3 elements table
Ions of Period 3 elements with increasing positive charge (metals) and increasing of outer electrons across the period
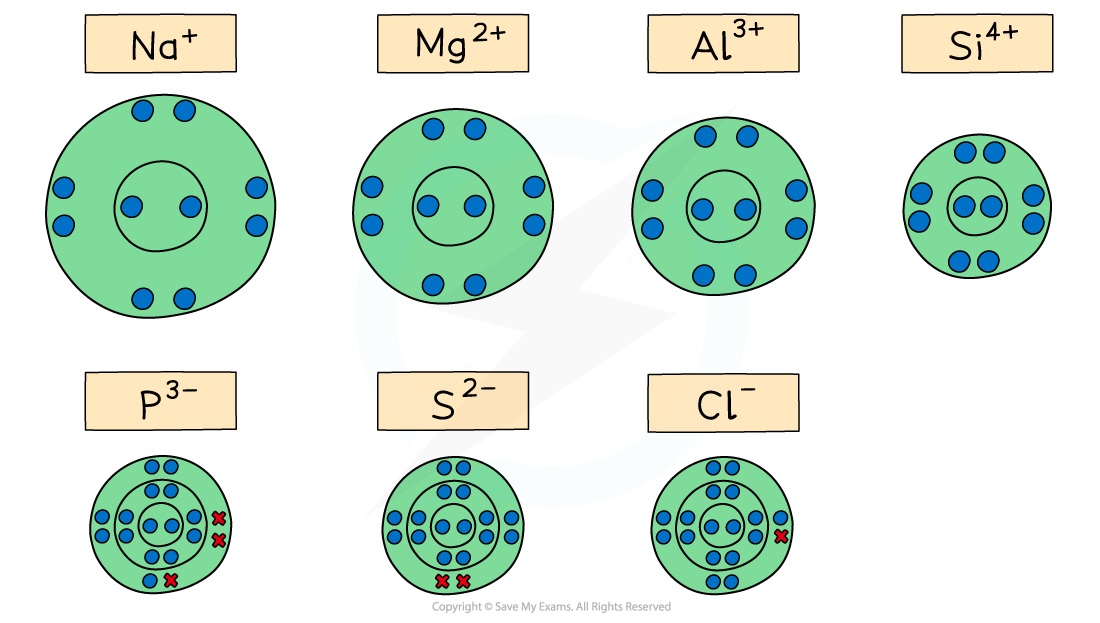
The diagram shows the electron configuration of the ions of Period 3 elements and their relative sizes
Melting point
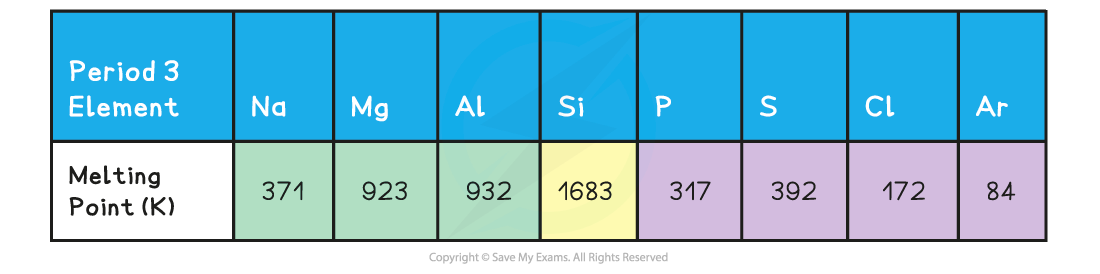
Melting points of the elements across Period 3 table
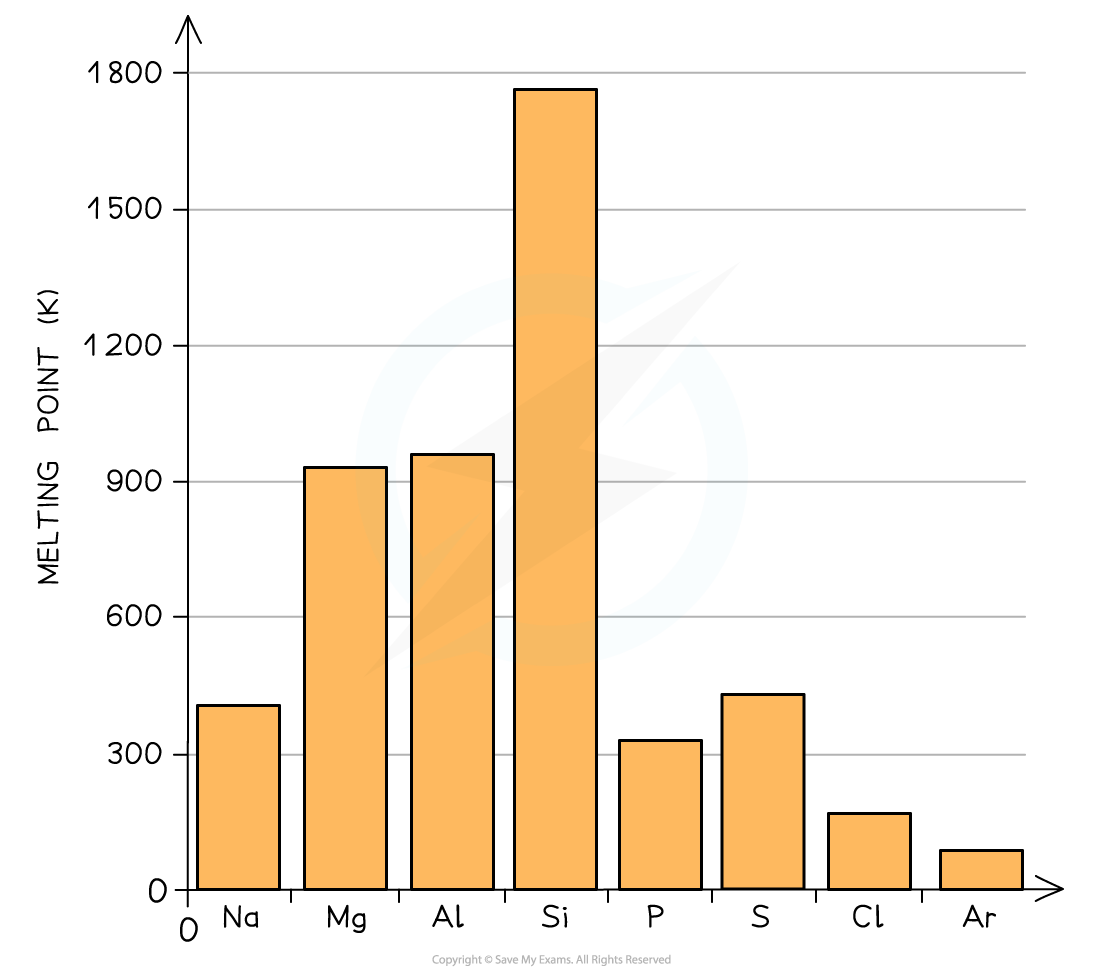
Ions of Period 3 elements with increasing positive charge (metals) and increasing of outer electrons across the period
- A general increase in melting point for the Period 3 elements up to silicon is observed
- Silicon has the highest melting point
- After the Si element the melting points of the elements decreases significantly
Electrical conductivity
- Electrical conductivity refers to how well a substance can conduct electricity
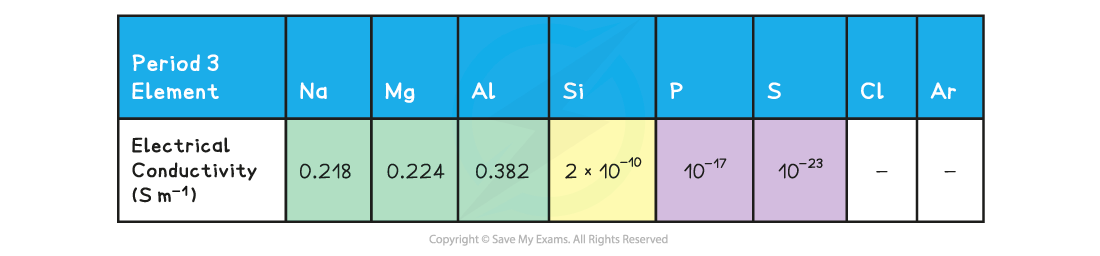
- Unlike the melting points, the electrical conductivity of the Period 3 elements shows a clear trend
- Going across the period, the electrical conductivity of the elements decreases significantly
Trends in electrical conductivity across Period 3 table
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









