- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记1.7.10 pH Scale
The pH Scale
- The pH scale is a numerical scale that shows how acidic or alkaline a solution is
- The values on the pH scale go from 1-14 (extremely acidic substances have values of below 1)
- All acids have pH values of below 7, all alkalis have pH values above 7
- The lower the pH then the more acidic the solution is
- The higher the pH then the more alkaline the solution is
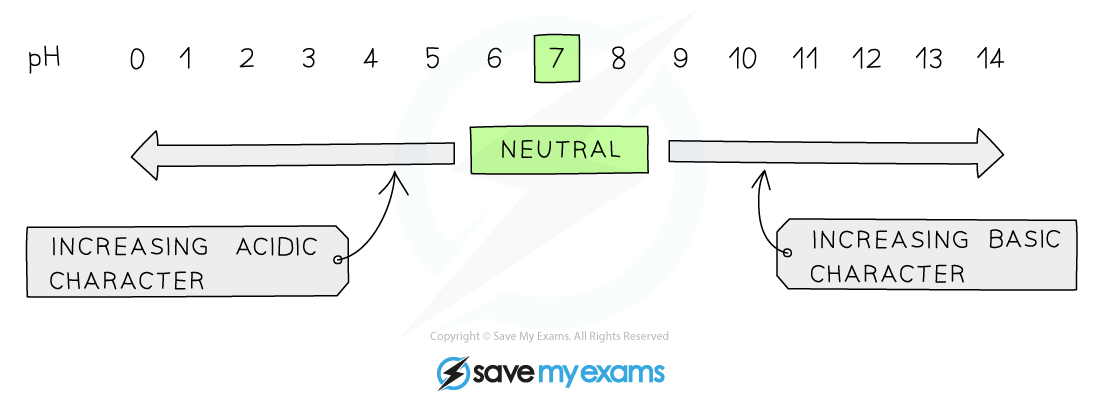
The pH scale showing acidity, neutrality and alkalinity
pH of water
- An equilibrium exists in water where few water molecules dissociate into proton and hydroxide ions
H2O(l) ⇌ H+(aq) + OH-(aq)
- The equilibrium constant for this reaction is:
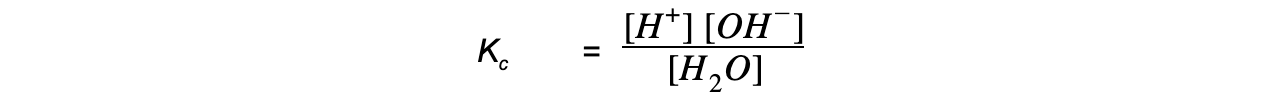
Kc x [H2O] = [H+] [OH-]
- Since the concentration the H+ and OH- ions is very small, the concentration of water is considered to be a constant, such that the expression can be rewritten as:
Kw = [H+] [OH-]
Where Kw (ionic product of water) = Kc x [H2O]
= 10-14 mol2 dm-3 at 298K
- Water at 298K has equal amounts of OH- and H+ ions with concentrations of 10-7 mol dm-3
- To calculate the pH of water, the following formula should be used:
 pH = -log (10-7)
pH = -log (10-7)
= 7
- Thus, water has a pH of 7
pH of acids
- Acidic solutions (strong or weak) always have more H+ than OH- ions
- Since the concentration of H+ is always greater than the concentration of OH- ions, [H+] is always greater than 10-7 mol dm-3
- Using the pH formula, this means that the pH of acidic solutions is always below 7
- The higher the [H+] of the acid, the lower the pH
pH of bases
- Basic solutions (strong or weak) always have more OH-than H+ ions
- Since the concentration of OH-is always greater than the concentration of H+ ions, [H+] is always smaller than 10-7 mol dm-3
- Using the pH formula, this means that the pH of basic solutions is always above 7
- The higher the [OH-] of the base, the higher the pH
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








