- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记1.7.7 Acids & Bases
Common Acids
- An acid is a substance that neutralises a base forming a salt and water:
2HCl(aq) + CaO(s) ⇌ CaCl2(aq) + H2O(l)
acid salt

Acids react with bases to form salt and water
- Acids are also substances that release hydrogen ions when they dissolve in water:
HCl(g) → H+(aq) + Cl- (aq)
 Acids dissociate in water to release a hydrogen ion
Acids dissociate in water to release a hydrogen ion
Names & formulae of some common acids table
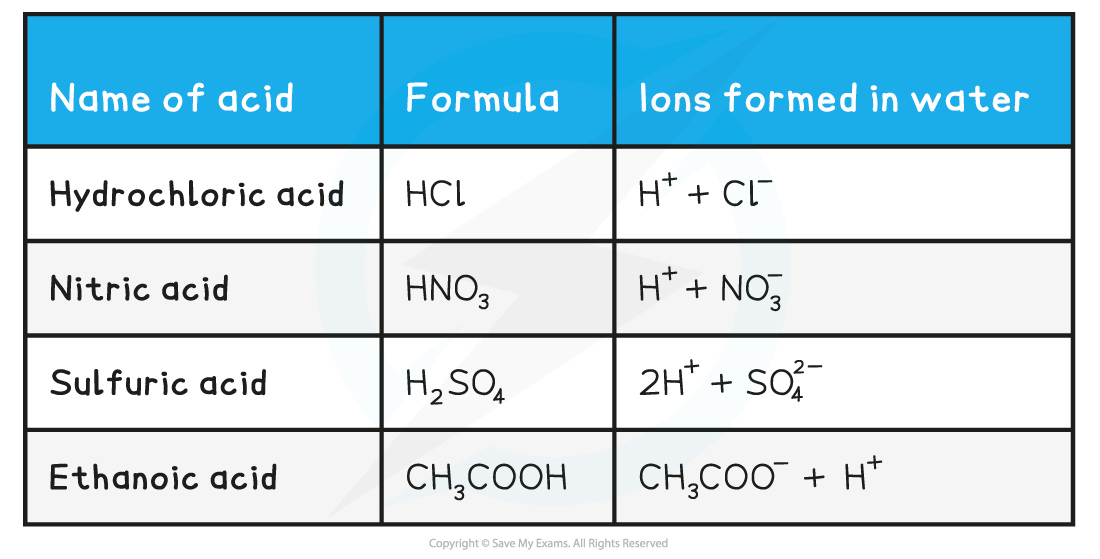
- In organic acids (such as carboxylic acids) only some of the hydrogen atoms can form ions when the acid dissociates
Common Alkalis
- A base is a compound that neutralises an acid forming a salt and water
2HCl(aq) + CaO(s) ⇌ CaCl2(aq) + H2O(l)
base salt
- A base is a substance that accepts hydrogen ions or a compound that contains oxide or hydroxide ions
- For example:
NH3(g) + H2O(l) → NH4+(aq) + OH-(aq)
base hydroxide ions
For example:
NaOH(s) + aq → Na+(aq) + OH-(aq)
base hydroxide ions
- A base that is soluble in water is called an alkali
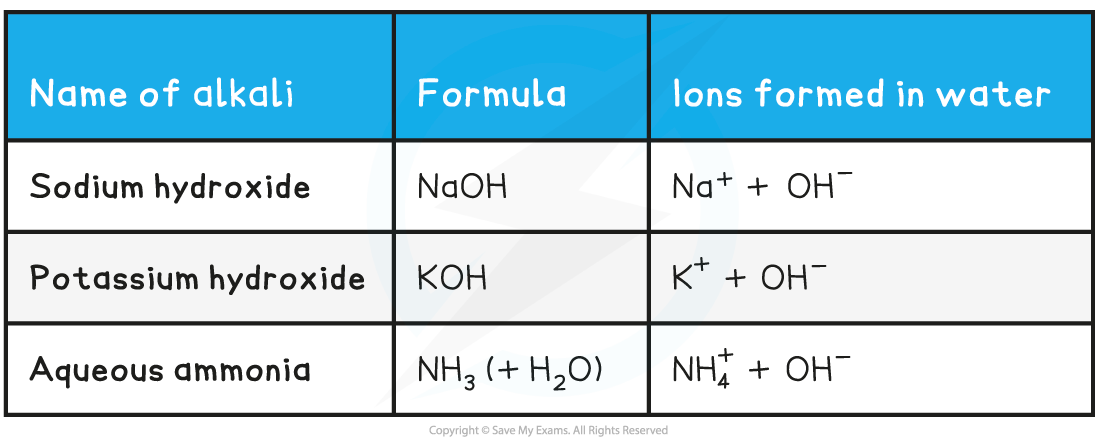
Names & formulae of some common alkalis table
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









