- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记1.7.3 Equilibrium Constant
Equilibrium Constant: Concentrations
Equilibrium expression & constant
- The equilibrium expression is an expression that links the equilibrium constant, Kc, to the concentrations of reactants and products at equilibrium taking the stoichiometry of the equation into account
- So, for a given reaction:
aA + bB ⇌ cC + dD
the Kc is defined as follows:
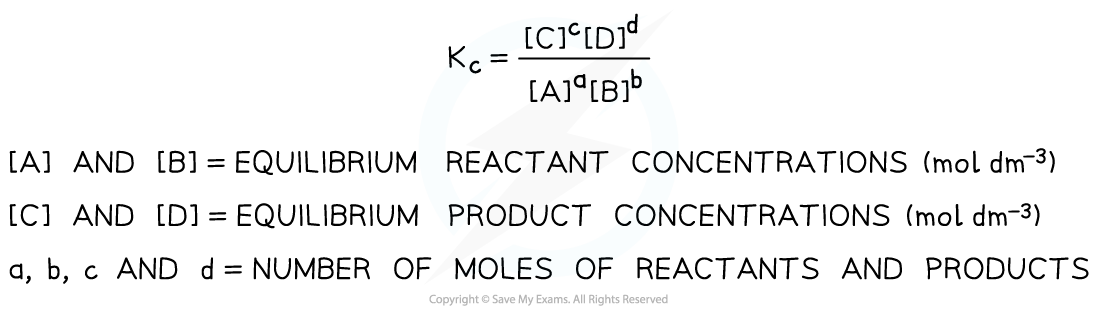
Equilibrium expression linking the equilibrium concentration of reactants and products at equilibrium
- Solids are ignored in equilibrium expressions
- The Kc of a reaction is specific and only changes if the temperature of the reaction changes
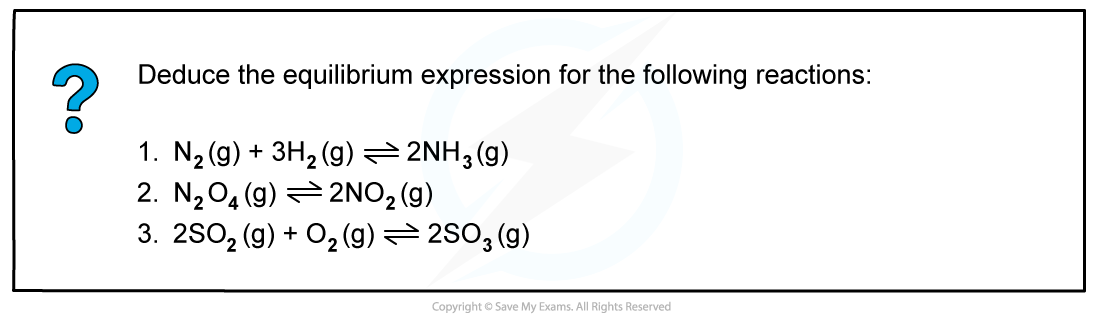
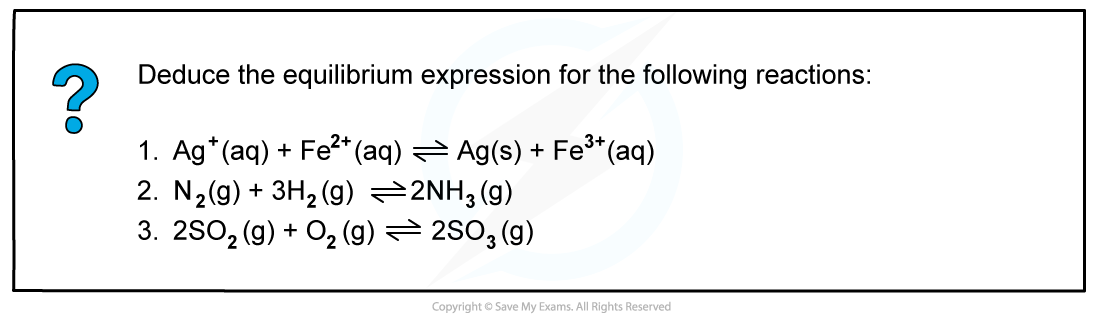
Worked example: Deducing equilibrium expressions
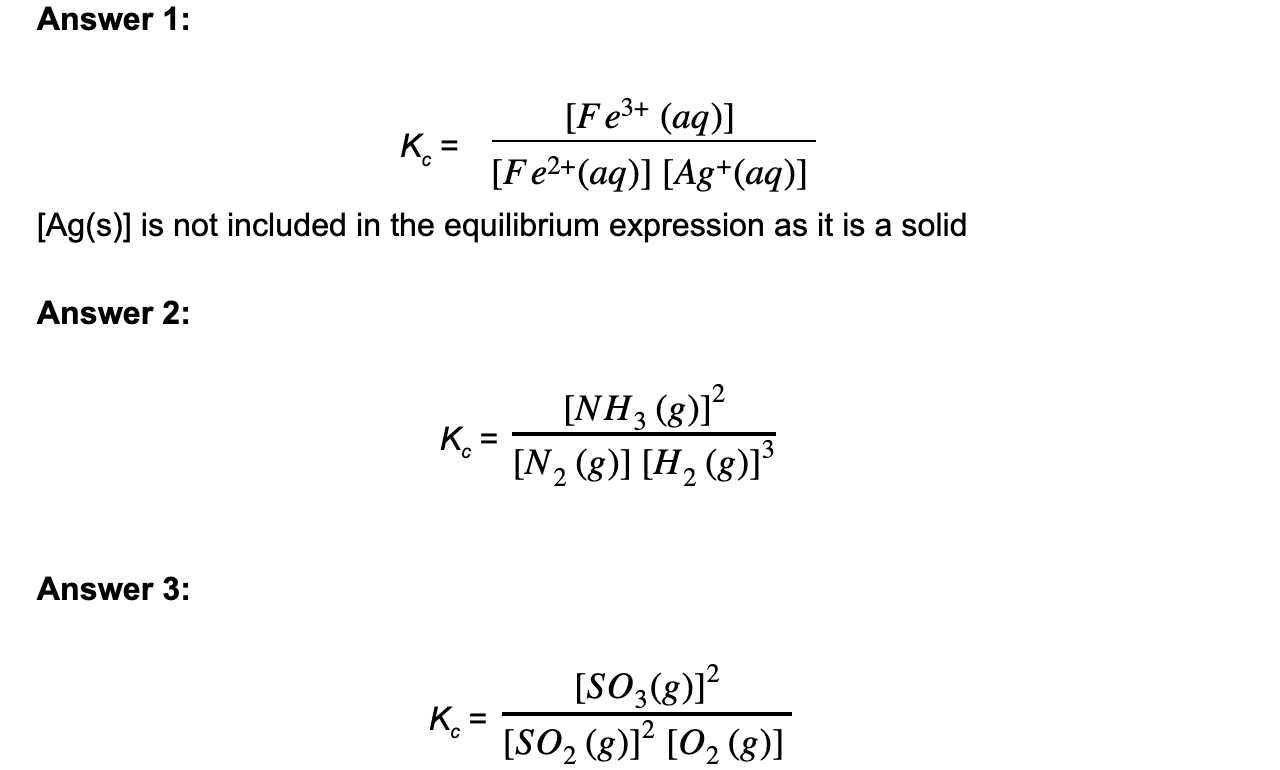
Answer
Mole Fraction & Partial Pressure
Partial pressure
- For reactions involving mixtures of gases, the equilibrium constant Kp is used as it is easier to measure the pressure than the concentration for gases
- The partial pressure of a gas is the pressure that the gas would have if it was in the container all by itself
- The total pressure is the sum of the partial pressure

Equation to calculate the total pressure in a mixture of gases
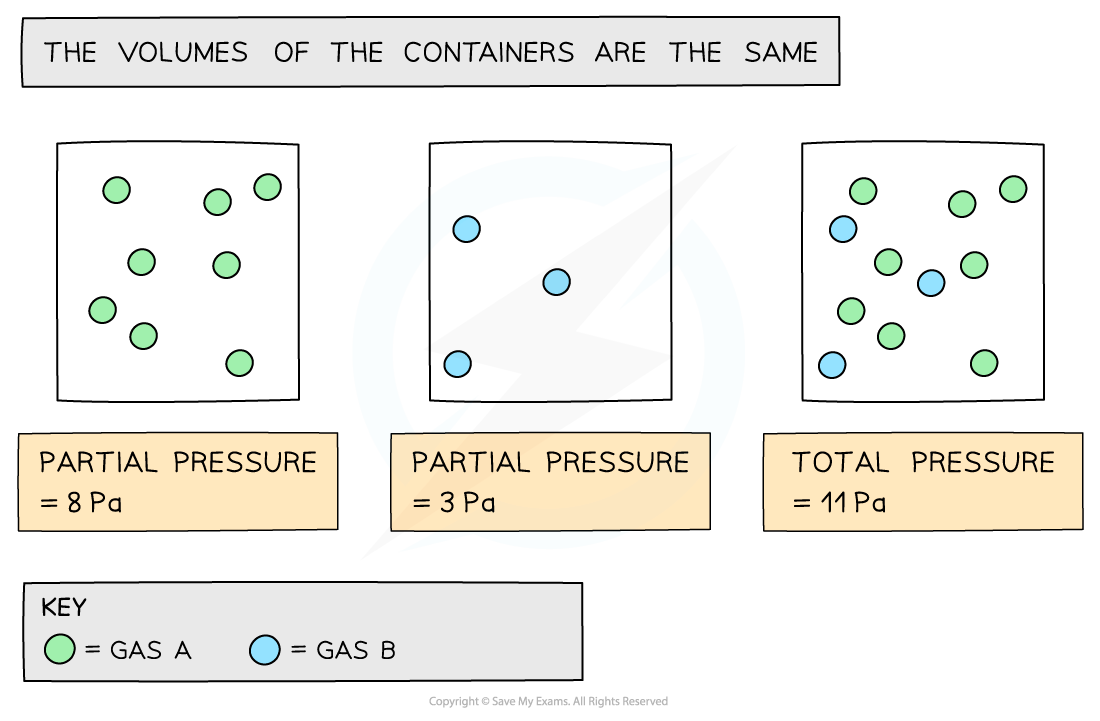 The diagram shows that each gas in the mixture has a partial pressure contributing towards the total pressure
The diagram shows that each gas in the mixture has a partial pressure contributing towards the total pressure
Mole fraction
- The mole fraction of a gas is the ratio of moles of a particular gas to the total number of moles of gas present
 Equation to calculate the mole fraction of a particular gas in a gas mixture
Equation to calculate the mole fraction of a particular gas in a gas mixture
- To calculate the partial pressures of each gas the following relationship can be used:
 Equation to calculate the partial pressure of a particular gas in a gas mixture
Equation to calculate the partial pressure of a particular gas in a gas mixture
The sum of the mole fractions should add up to 1.00, while the sum of the partial pressures should add up to the total pressure.
Equilibrium Constant: Partial Pressures
Equilibrium expressions involving partial pressures
- Equilibrium expressions in terms of partial pressures are written similarly to those involving concentrations with a few differences:
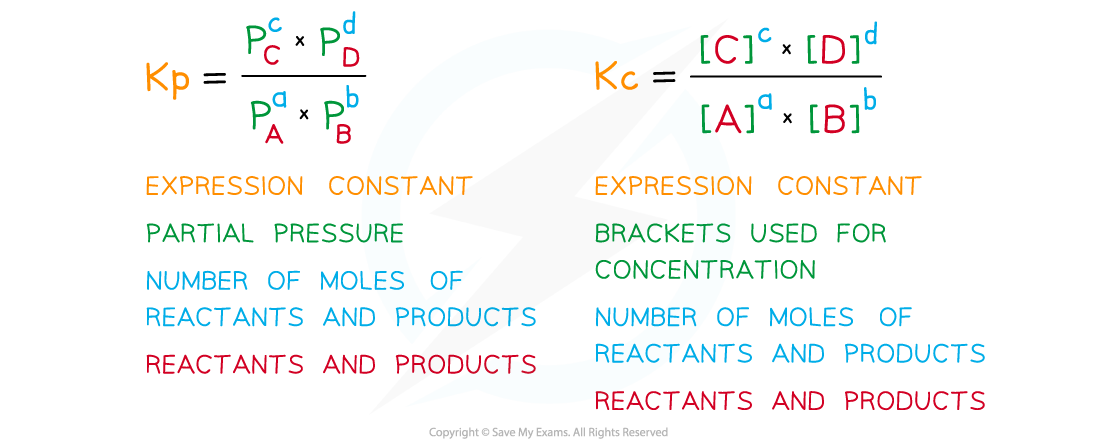 The diagram shows a comparison of writing equilibrium expressions in terms of partial pressures and concentrations
The diagram shows a comparison of writing equilibrium expressions in terms of partial pressures and concentrations
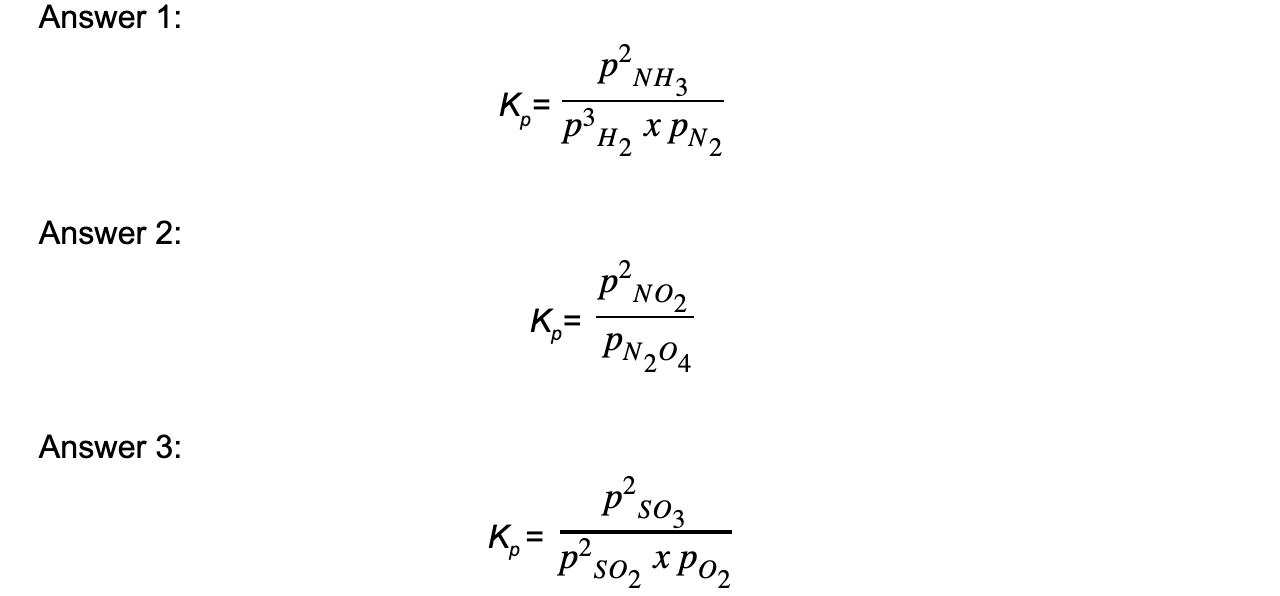
Worked example: Deducing equilibrium expressions of gaseous reactions
Answer
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1