- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记1.6.3 Oxidising & Reducing Agents
Oxidising & Reducing Agents
Oxidising agent
- An oxidising agent is a substance that oxidises another atom or ion by causing it to lose electrons
- An oxidising agent itself gets reduced – gains electrons
- Therefore, the ox. no. of the oxidising agent decreases

Example of an oxidising agent in a chemical reaction
Reducing agent
- A reducing agent is a substance that reduces another atom or ion by causing it to gain electrons
- A reducing agent itself gets oxidised – loses/donates electrons
- Therefore, the ox. no. of the reducing agent increases

Example of a reducing agent in a chemical reaction
- For a reaction to be recognised as a redox reaction, there must be both an oxidising and reducing agent
- Some substances can act both as oxidising and reducing agents
- Their nature is dependent upon what they are reacting with and the reaction conditions
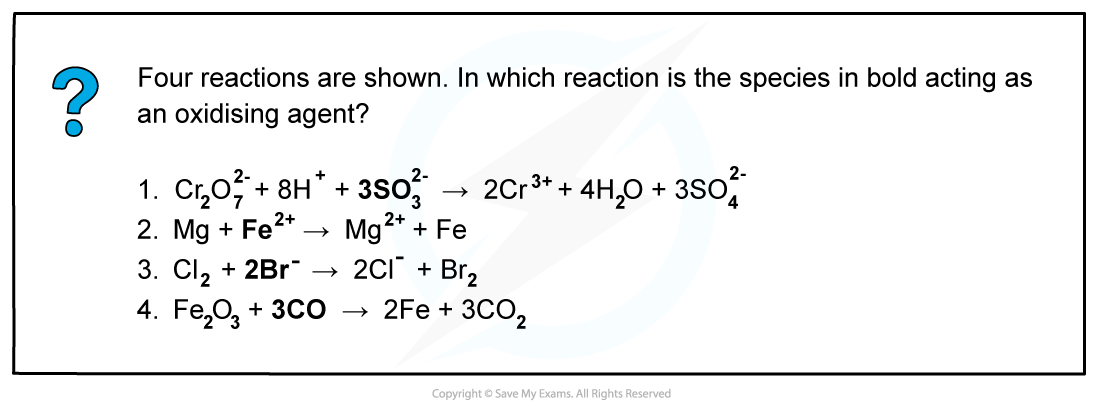
Worked Example: Oxidising & reducing agents
Answer
Oxidising agents are substances that oxidise other species, gain electrons and are themselves reduced.
Write down the oxidation numbers of each species in the reaction
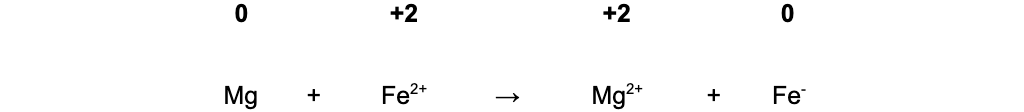
In equation B, Fe2+ oxidises Mg(0) to Mg2+(+2) and is itself reduced from Fe2+(+2) to Fe(0)
Roman numerals
- Roman numerals are used to show the oxidation states of transition metals which can have more than one oxidation number
- Iron can be both +2 and +3 so Roman numerals are used to distinguish between them
- Fe2+ in FeO can be written as Iron(II) oxide
- Fe3+ in Fe2O3 can be written as Iron(III) oxide
Worked example: Systematic names of compounds
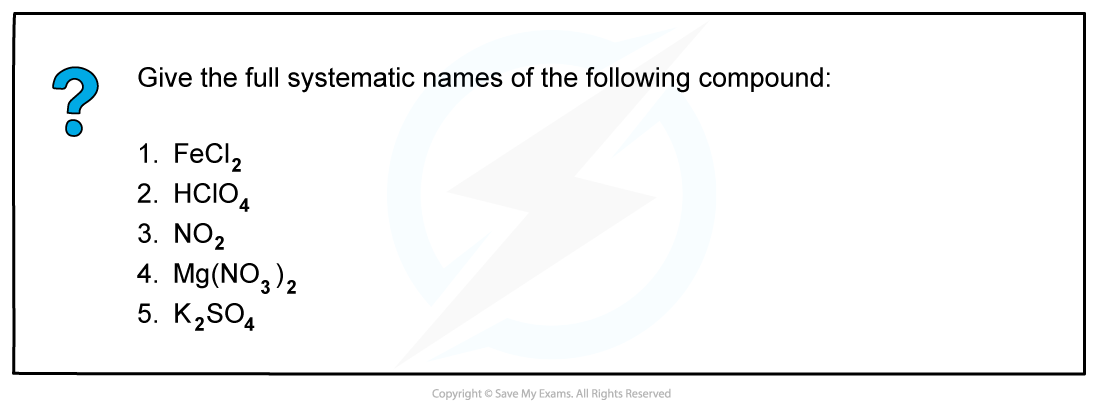
Answer
Answer 1: Iron(II) chloride: ox. no. of 2 Cl atoms is -2 and FeCl2 has overall no charge so ox. no. of Fe is +2
Answer 2: Chloric(VII) acid: ox. no. of H is +1, 4 O atoms is -8 and HClO4 has overall no charge so ox. no. of Cl is +7
Answer 3: Nitrogen(IV) oxide: ox. no. of 2 O atoms is -4 and NO2 has overall no charge so ox. no. of N is +4
Answer 4: Magnesium nitrate: this is a salt of the common acid, so they are named without including the ox. no. of the non-metal
Answer 5: Potassium sulfate: this is a salt of the common acid, so they are named without including the ox. no. of the non-metal
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1