- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记1.5.3 Standard Enthalpy Change
Enthalpy Changes at Standard Conditions
- To fairly compare the changes in enthalpy between reactions, all reactions should be carried out under standard conditions
- These standard conditions are:
- A pressure of 101 kPa
- A temperature of 298 K (25 oC)
- Each substance involved in the reaction is in its normal physical state (solid, gas or liquid)
- To show that a reaction has been carried out under standard conditions, the symbol Ꝋ is used
- ΔHꝊ = the standard enthalpy change
- These are a number of key definitions for common language relating to enthalpy change that all chemists need to know
Enthalpy definitions table

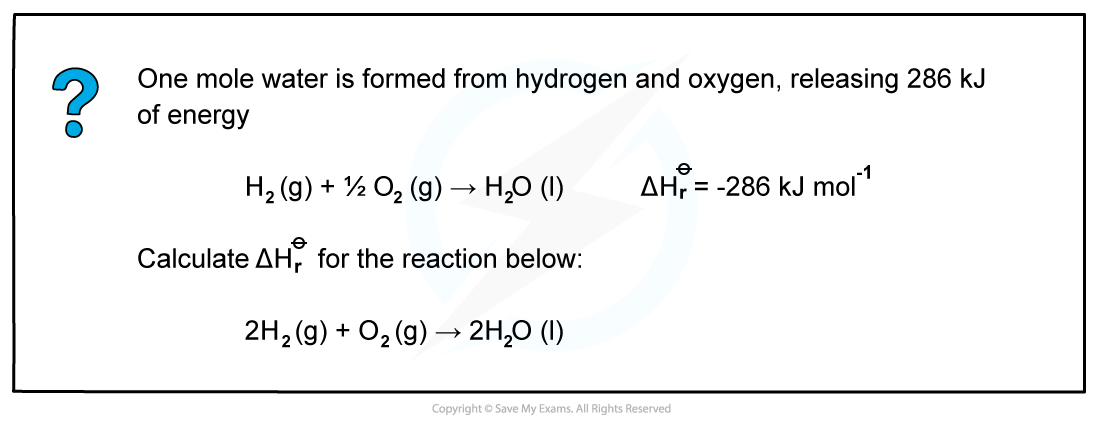
Worked example: Calculating the enthalpy change of reaction of water
Answer
Since two moles of water molecules are formed in the question above, the energy released is simply:
ΔHrꝊ = 2 mol x (-286 kJ mol-1)= -572 kJ mol-1
Worked example: Calculating the enthalpy change of formation

Answer
Since two moles of Fe2O3 (s) are formed the total change in enthalpy for the reaction above is:
ΔHfꝊ = 2 x ( -824.2 kJ mol-1)= - 1648 kJ
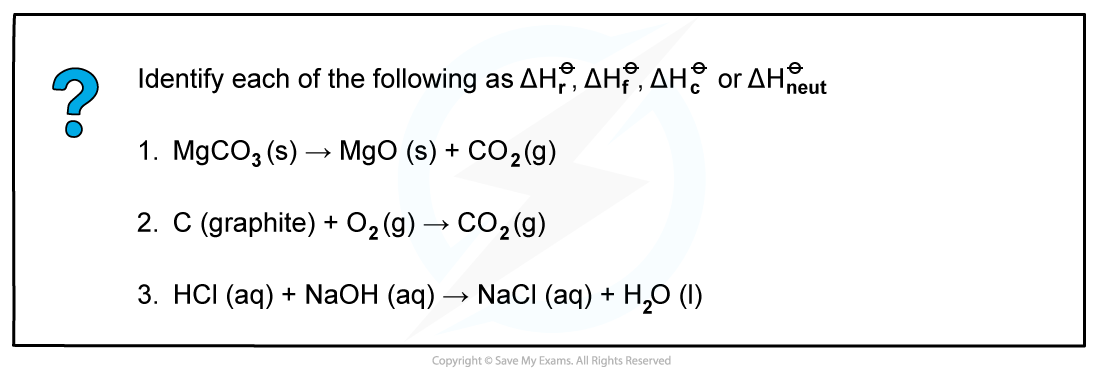
Worked example: Calculating enthalpy changes
Answer
Answer 1: ΔHrꝊ
Answer 2: ΔHrꝊ as one mole of CO2 is formed from its elements in standard state and ΔHcꝊ as one mole of carbon is burnt in oxygen
Answer 3: ΔHneutꝊ as one mole of water is formed from the reaction of an acid and alkali
Exam Tip
The ΔHfꝊ of an element in its standard state is zero.For example, ΔHfꝊof O2(g) is 0 kJ mol-1
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









