- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记1.4.2 Ideal Gas Law & Equation
Gases: Ideal Gas Law & Equation
Kinetic theory of gases
- The kinetic theory of gases states that molecules in gases are constantly moving
- The theory makes the following assumptions:
- The gas molecules are moving very fast and randomly
- The molecules hardly have any volume
- The gas molecules do not attract or repel each other (no intermolecular forces)
- No kinetic energy is lost when the gas molecules collide with each other (elastic collisions)
- The temperature of the gas is related to the average kinetic energy of the molecules
- Gases that follow the kinetic theory of gases are called ideal gases
- However, in reality gases do not fit this description exactly but may come very close and are called real gases
Ideal gases
- The volume that an ideal gas occupies depends on:
- Its pressure
- Its temperature
- See section Changing gas volume of page Gas Pressure
- When a gas is heated (at constant pressure) the particles gain more kinetic energy and undergo more frequent collisions with the container wall
- To keep the pressure constant, the molecules must get further apart and therefore the volume increases
- The volume is therefore directly proportional to the temperature (at constant pressure)
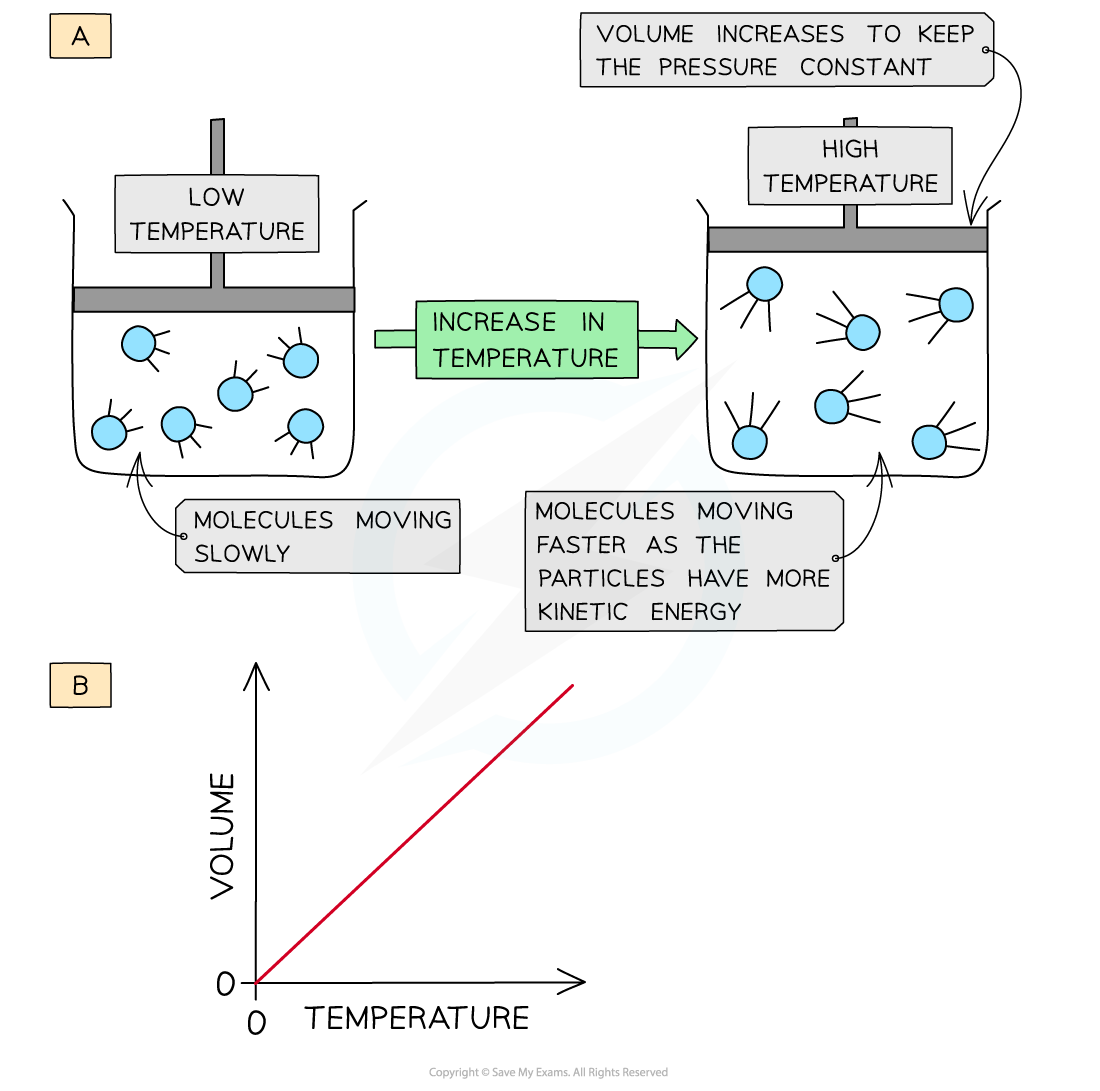
The volume of a gas increases upon heating to keep a constant pressure (a); volume is directly proportional to the pressure (b)
Limitations of the ideal gas law
- At very high pressures and low temperatures real gases do not obey the kinetic theory as under these conditions:
- Molecules are close to each other
- There are instantaneous dipole- induced dipole or permanent dipole- permanent dipole forces between the molecules
- These attractive forces pull the molecules away from the container wall
- The volume of the molecules is not negligible
- Real gases therefore do not obey the following kinetic theory assumptions at high temperatures and pressures:
- There is zero attraction between molecules (due to attractive forces, the pressure is lower than expected for an ideal gas)
- The volume of the gas molecules can be ignored (volume of the gas is smaller than expected for an ideal gas)
Ideal gas equation
- The ideal gas equation shows the relationship between pressure, volume, temperature and number of moles of gas of an ideal gas:
pV = nRT
p = pressure (pascals, Pa)
V = volume (m3)
n = number of moles of gas (mol)
R = gas constant (8.31 J K-1 mol-1)
T = temperature (kelvin, K)
- The ideal gas equation can also be used to calculate the molar mass (Mr) of a gas
Worked example: Calculating the volume of a gas
Answer
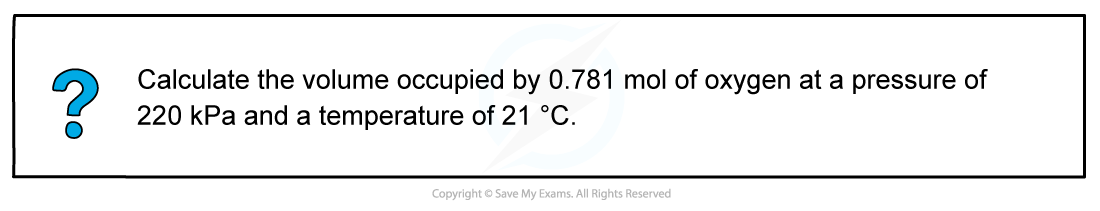
- Step 1: Rearrange the ideal gas equation to find volume of gas
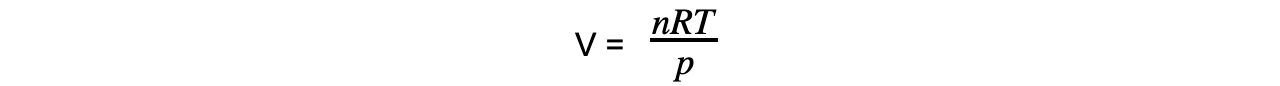
- Step 2: Calculate the volume the oxygen gas occupies
p = 220 kPa = 220 000 Pa
n = 0.781 mol
R = 8.31 J K-1 mol-1
T = 21oC = 294 K
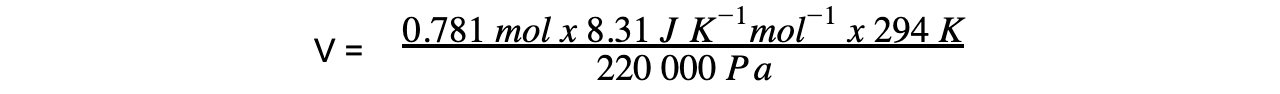
= 0.00867 m3
= 8.67 dm3
Worked example: Calculating the molar mass of a gas
Answer
- Step 1: Rearrange the ideal gas equation to find the number of moles of gas
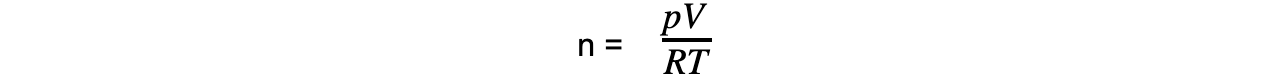
- Step 2: Calculate the number of moles of gas
p = 300 kPa = 300 000 Pa
V = 1000 cm3 = 0.001 m3
R = 8.31 J K-1 mol-1
T = 23 oC = 296 K
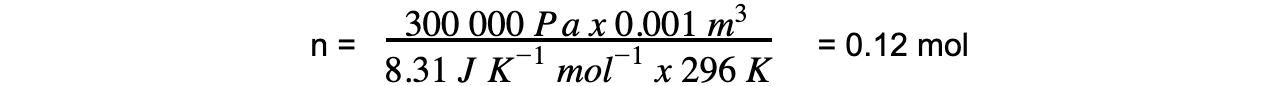
- Step 3: Calculate the molar mass using the number of moles of gas
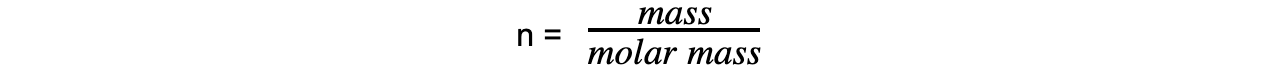
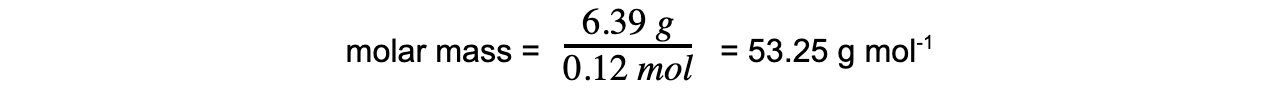
Exam Tip
Ideal gases have zero particle volume (the particles are really small) and no intermolecular forces of attraction or repulsion.To calculate the temperature in Kelvin, add 273 to the Celsius temperature, eg. 100 oC is 373 Kelvin. Remember: an ideal gas will have a volume that is directly proportional to the temperature and inversely proportional to the pressure.
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









