- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记1.4.1 Gas Pressure
Gases: Gas Pressure
- Gases in a container exert a pressure as the gas molecule are constantly colliding with the wall of the container
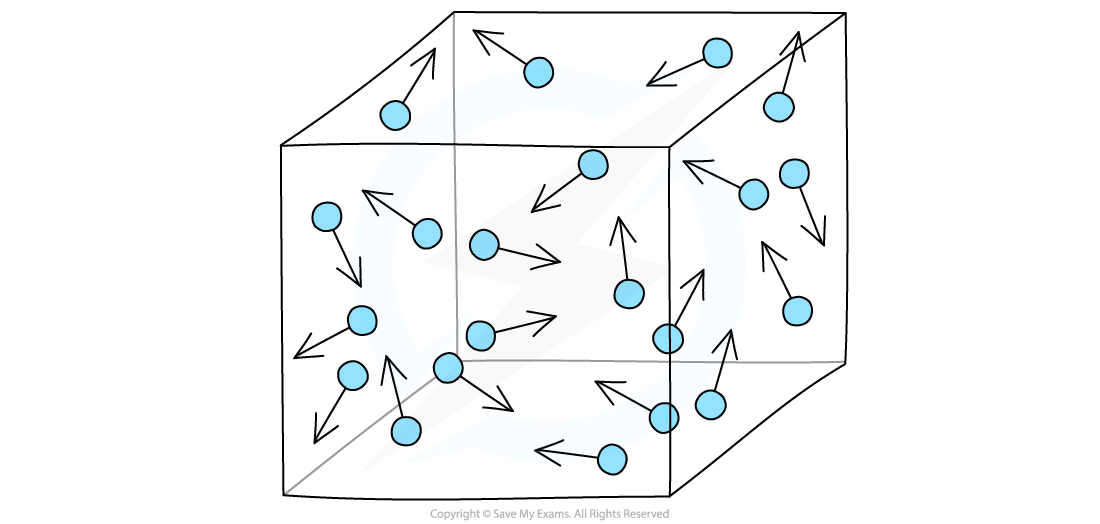
Gas particles exert a pressure by constantly colliding with the walls of the container
Changing gas volume
- Decreasing the volume (at constant temperature) of the container causes the molecules to be squashed together which results in more frequent collisions with the container wall
- The pressure of the gas increases
- The volume is therefore inversely proportional to the pressure (at constant temperature)
- A graph of volume of gas plotted against 1/pressure gives a straight line
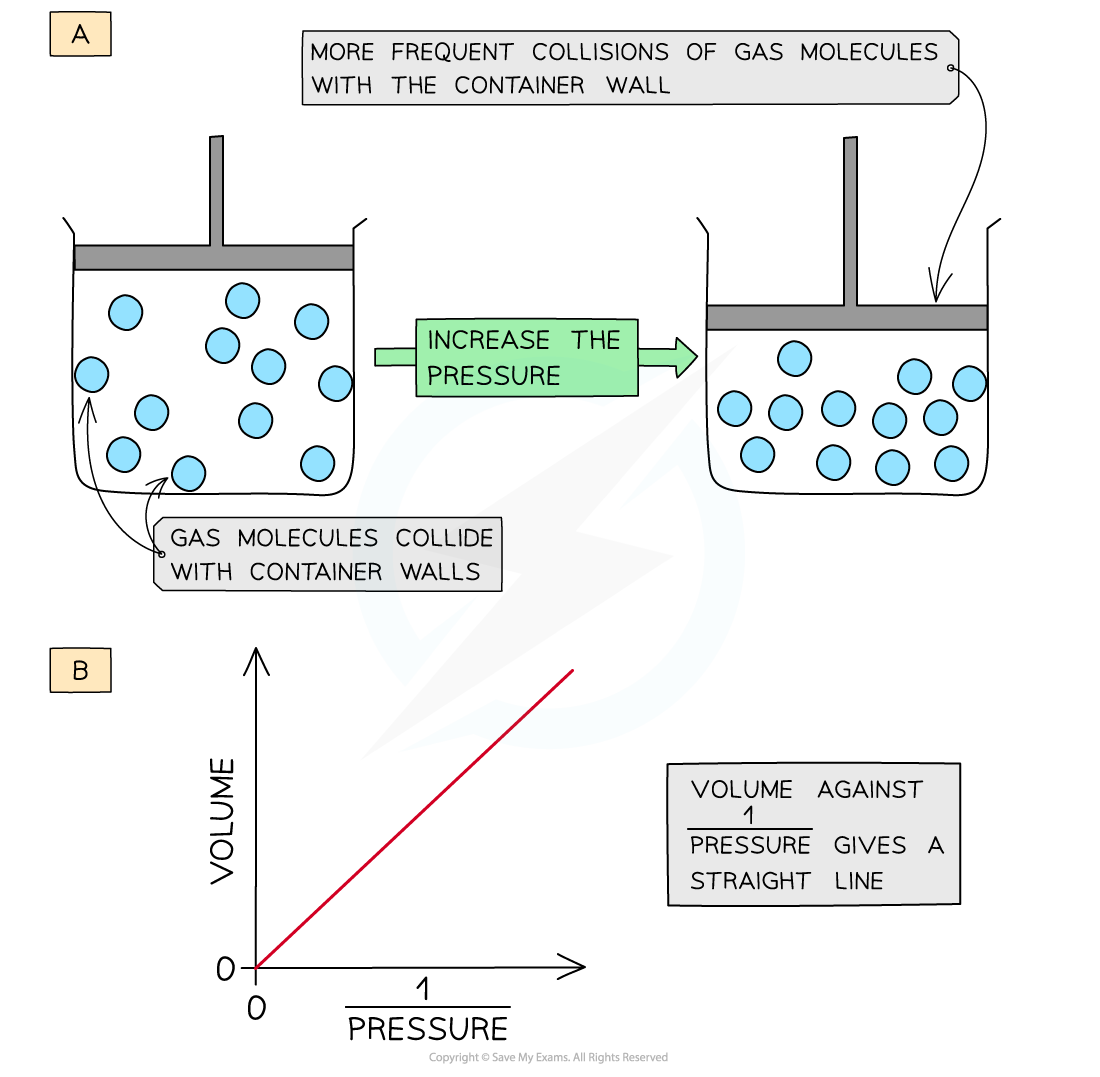
Decreasing the volume of a gas causes an increased collision frequency of the gas particles with the container wall (a); volume is inversely proportional to the pressure (b)
Changing gas temperature
- Increasing the temperature (at constant volume) of the gas causes the molecules to gain more kinetic energy
- This means that the particles will move faster and collide with the container walls more frequently
- The pressure of the gas increases
- The temperature is therefore directly proportional to the pressure (at constant volume)
- A graph of temperature of gas plotted against pressure gives a straight line
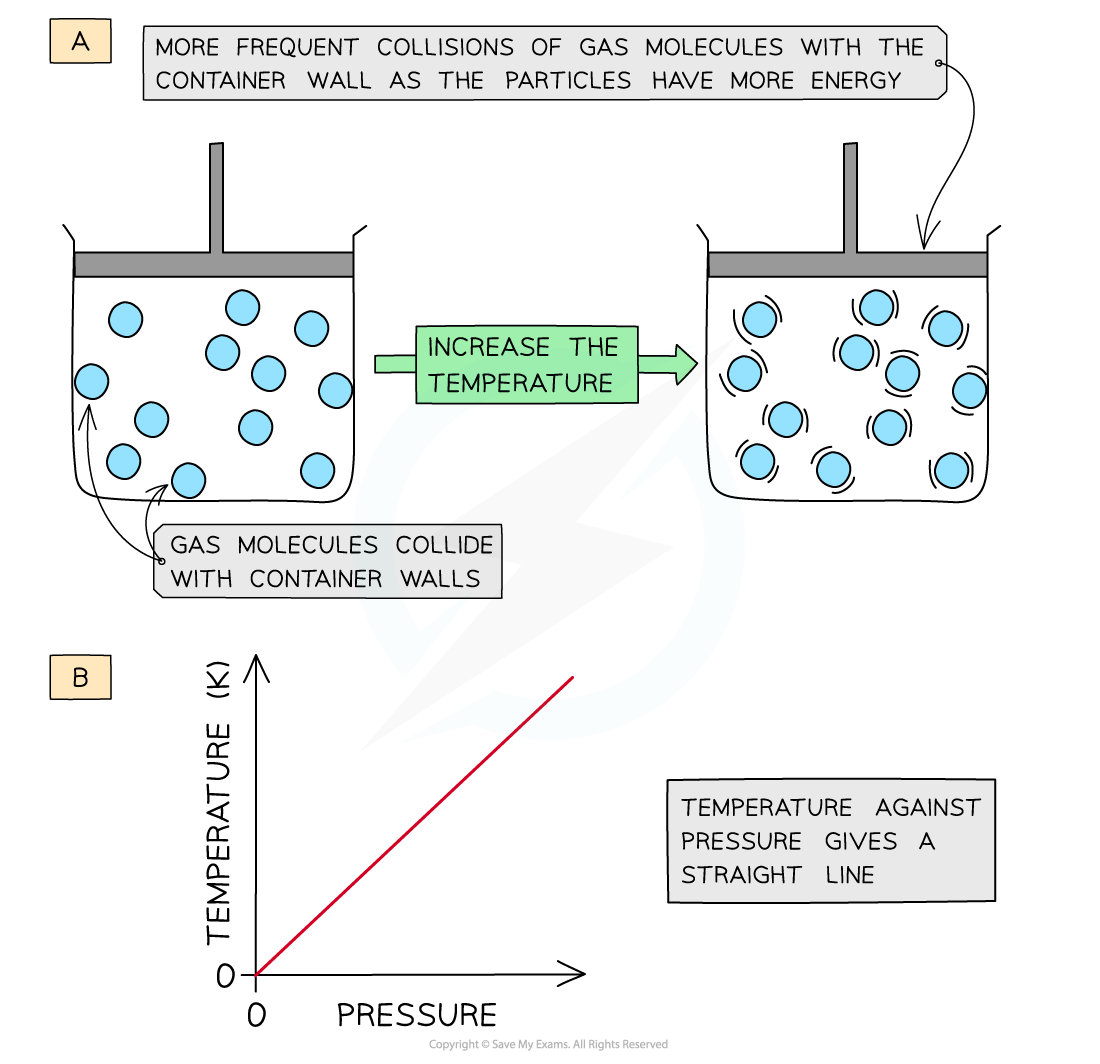
Increasing the temperature of a gas causes an increased collision frequency of the gas particles with the container wall (a); temperature is directly proportional to the pressure (b)
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









