- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记1.3.12 Hydrogen Bonding
Hydrogen Bonding
Hydrogen bonding
- Hydrogen bonding is the strongest form of intermolecular bonding
- Intermolecular bonds are bonds between molecules
- Hydrogen bonding is a type of permanent dipole – permanent dipole bonding
- For hydrogen bonding to take place the following is needed:
- A species which has an O or N (very electronegative) atom with an available lone pair of electrons
- A species with an -OH or -NH group
- When hydrogen is covalently bonded to an electronegative atom, such as O or N, the bond becomes very highly polarised
- The H becomes so δ+ charged that it can form a bond with the lone pair of an O or N atom in another molecule
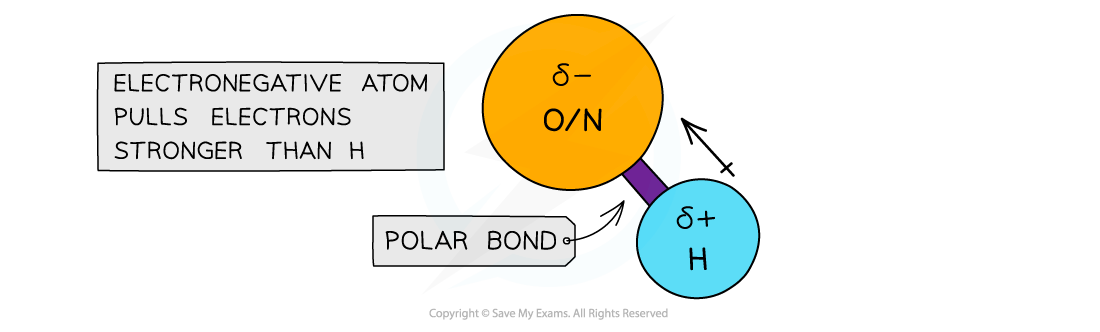
The electronegative atoms O or N have a stronger pull on the electrons in the covalent bond with hydrogen, causing the bond to become polarised
- For hydrogen bonding to take place, the angle between the -OH/-NH and the hydrogen bond is 180o
- The number of hydrogen bonds depends on:
- The number of hydrogen atoms attached to O or N in the molecule
- The number of lone pairs on the O or N
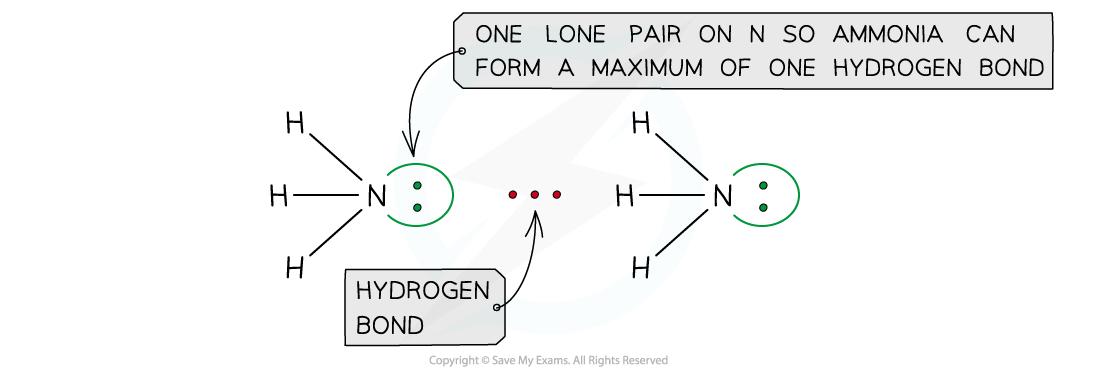
Ammonia can form a maximum of one hydrogen bond per molecule
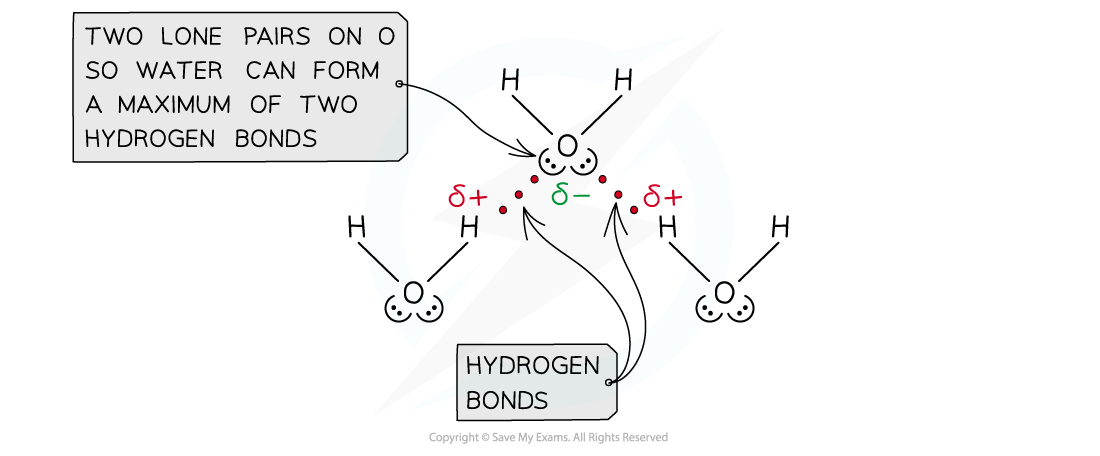
Water can form a maximum of two hydrogen bonds per molecule
Properties of water
- Hydrogen bonding in water, causes it to have anomalous properties such as high melting and boiling points, high surface tension and anomalous density of ice compared to water
High melting & boiling points
- Water has high melting and boiling points which is caused by the strong intermolecular forces of hydrogen bonding between the molecules
- In ice (solid H2O) and water (liquid H2O) the molecules are tightly held together by hydrogen bonds
- A lot of energy is therefore required to break the water molecules apart and melt or boil them
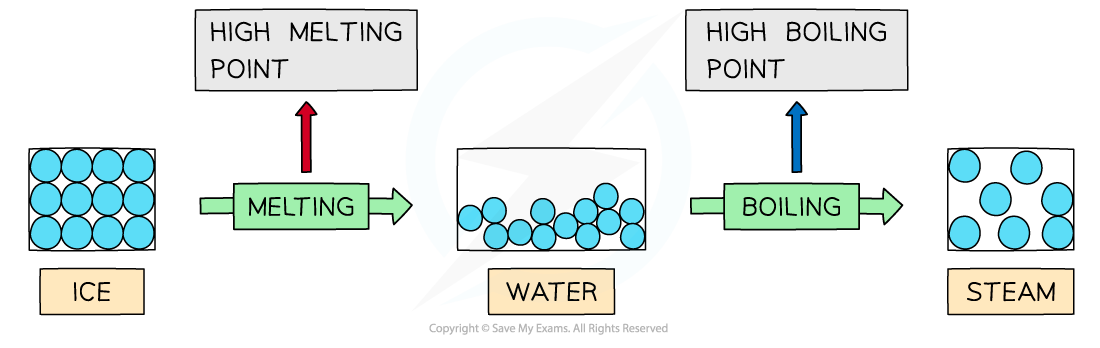 Hydrogen bonds are strong intermolecular forces which are difficult to break causing water to have high melting and boiling points
Hydrogen bonds are strong intermolecular forces which are difficult to break causing water to have high melting and boiling points
- The graph below compares the enthalpy of vaporisation (energy required to boil a substance) of different hydrides
- The enthalpy changes increase going from H2S to H2Te due to the increased number of electrons in the Group 16 elements
- This causes an increased instantaneous dipole - induced dipole forces as the molecules become larger
- Based on this, H2O would have a much lower enthalpy change (around 17 kJ mol-1)
- However, the enthalpy change of vaporisation is almost 3 times larger which is caused by the hydrogen bonds present in water but not in the other hydrides
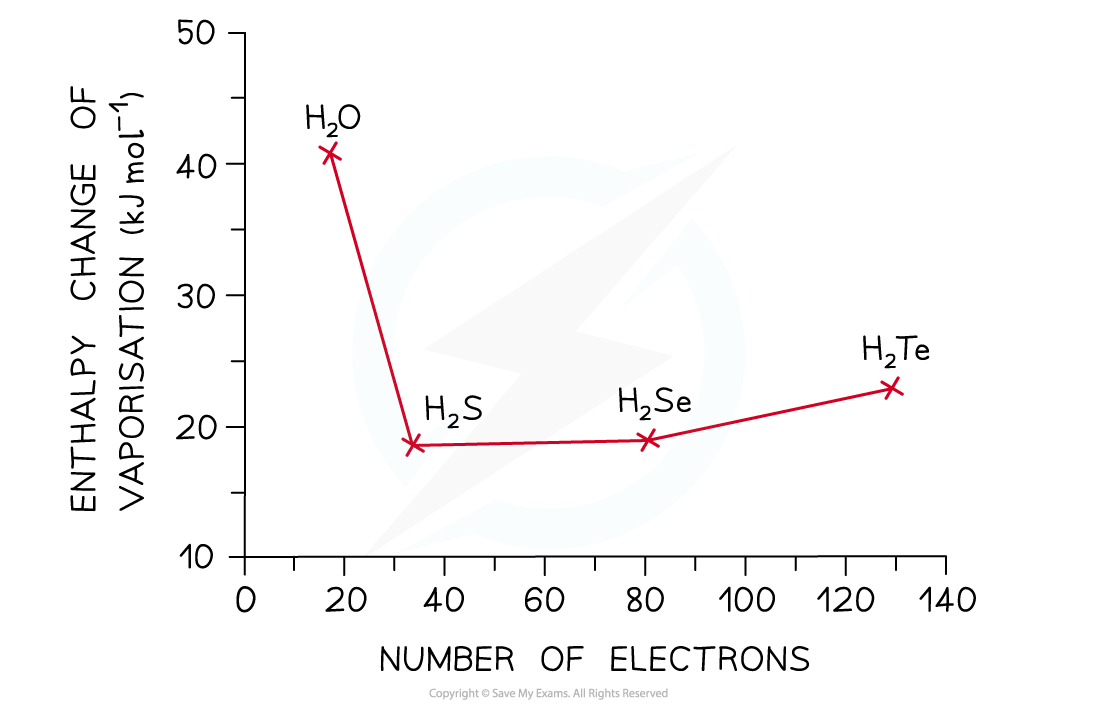
The high enthalpy change of evaporation of water suggests that instantaneous dipole-induced dipole forces are not the only forces present in the molecule – there are also those of the strong hydrogen bonds, which cause the high boiling points
High surface tension
- Water has a high surface tension
- Surface tension is the ability of a liquid surface to resist any external forces (i.e. to stay unaffected by forces acting on the surface)
- The water molecules at the surface of liquid are bonded to other water molecules through hydrogen bonds
- These molecules pull downwards on the surface molecules causing the surface them to become compressed and more tightly together at the surface
- This increases water’s surface tension
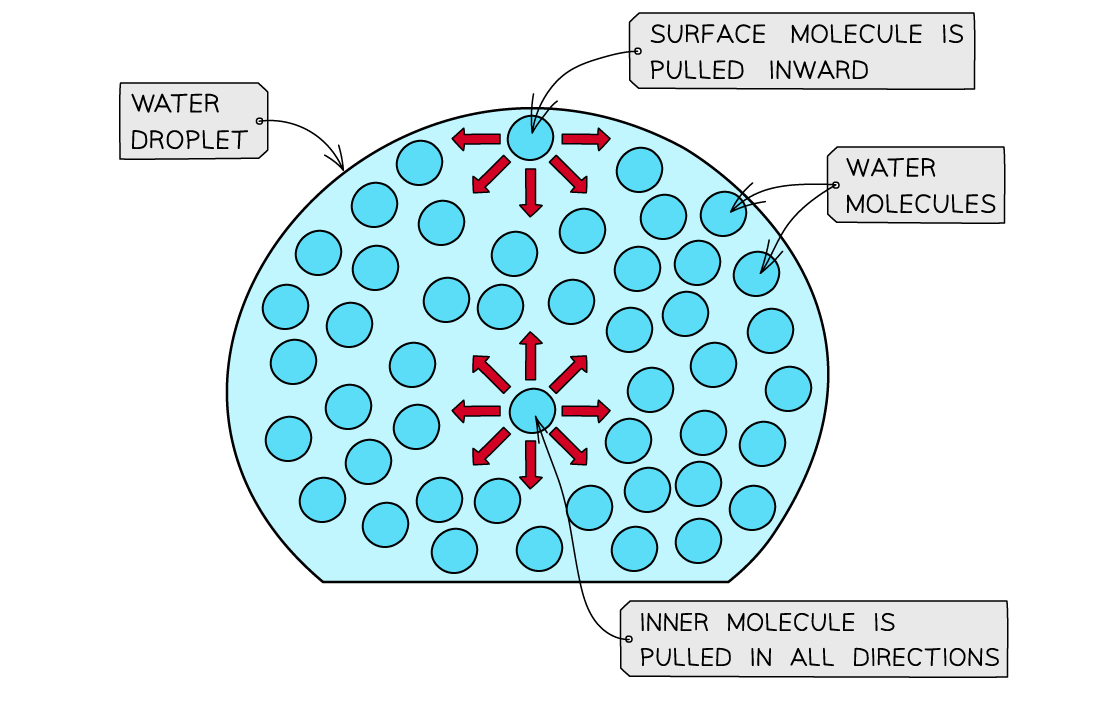
The surface molecules are pulled downwards due to the hydrogen bonds with other molecules, whereas the inner water molecules are pulled in all directions
Density
- Solids are denser than their liquids as the particles in solids are more closely packed together than in their liquid state
- In ice however, the water molecules are packed in a 3D hydrogen-bonded network in a rigid lattice
- Each oxygen atom is surrounded by hydrogen atoms
- This way of packing the molecules in a solid and the relatively long bond lengths of the hydrogen bonds means that the water molecules are slightly further apart than in the liquid form
- Therefore, ice has a lower density than liquid water
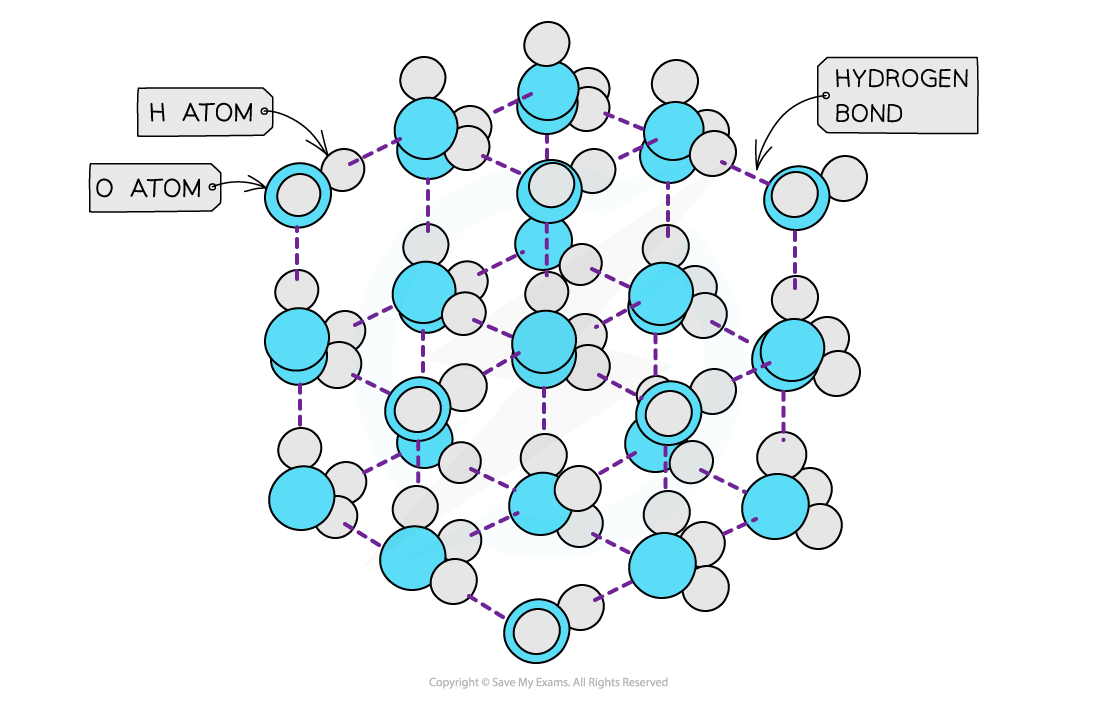
The ‘more open’ structure of molecules in ice causes it to have a lower density than liquid water
Exam Tip
Ice floats on water because of ice's lower density.
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









