- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记1.3.9 Hybridisation
Covalent Bonding: Orbitals & Hybridisation
Bond overlap in covalent bonds
- A single covalent bond is formed when two nonmetals combine
- Each atom that combines has an atomic orbital containing a single unpaired electron
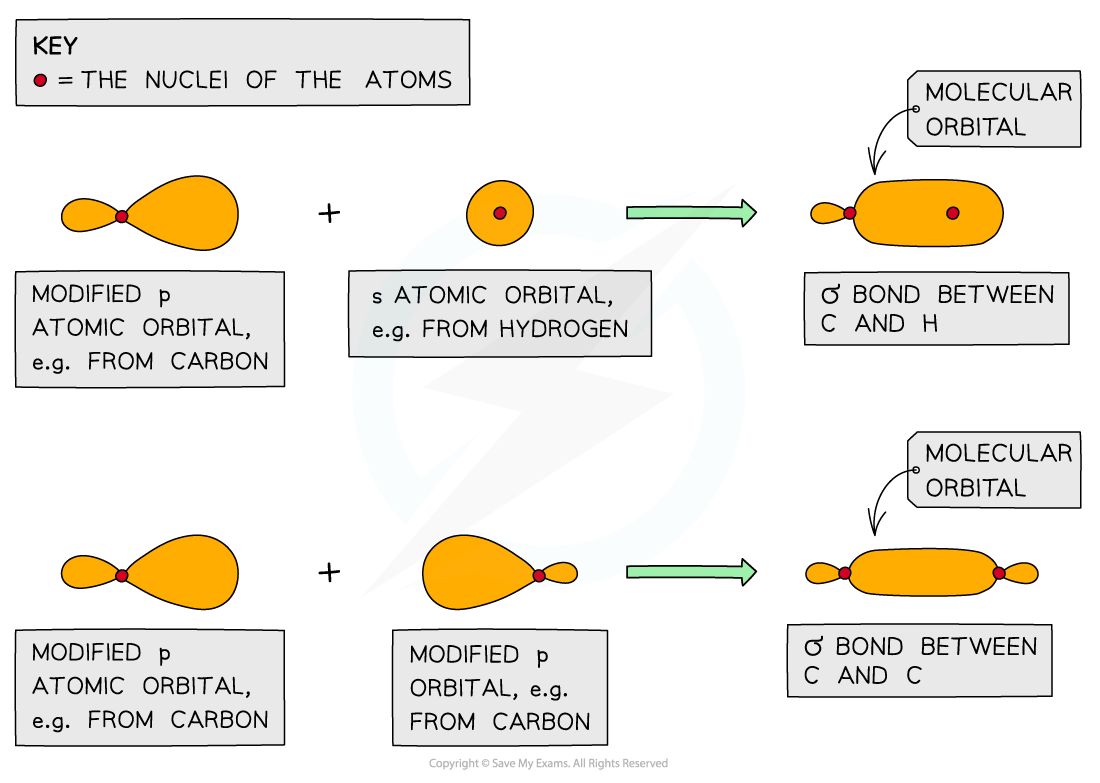
- When a covalent bond is formed, the atomic orbitals overlap to form a combined orbital containing two electrons
- This new orbital is called the molecular orbital
- The greater the atomic orbital overlap, the stronger the bond
- Sigma (σ) bonds are formed by direct overlap of orbitals between the bonding atoms
- Pi (π) bonds are formed by the sideways overlap of adjacent above and below the σ bond
σ bonds
- Sigma (σ) bonds are formed from the end-on overlap of atomic orbitals
- S orbitals overlap this way as well as p orbitals
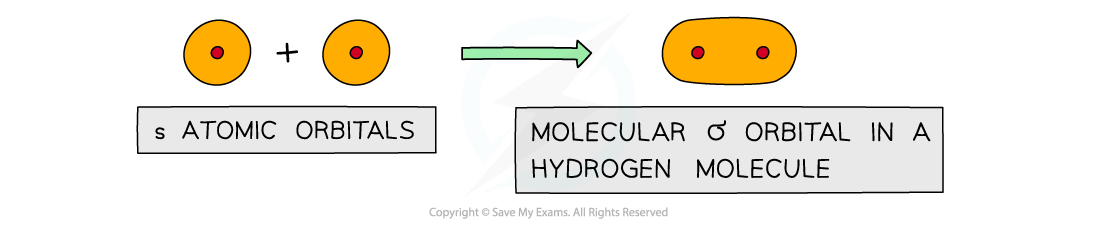
Sigma orbitals can be formed from the end-on overlap of s orbitals
- The electron density in a σ bond is symmetrical about a line joining the nuclei of the atoms forming the bond
- The pair of electrons is found between the nuclei of the two atoms
- The electrostatic attraction between the electrons and nuclei bonds the atoms to each other
π bonds
- Pi (π) bonds are formed from the sideways overlap of adjacent p orbitals
- The two lobes that make up the π bond lie above and below the plane of the σ bond
- This maximises overlap of the p orbitals
- A single π bond is drawn as two electron clouds one arising from each lobe of the p orbitals
- The two clouds of electrons in a π bond represent one bond containing two electrons
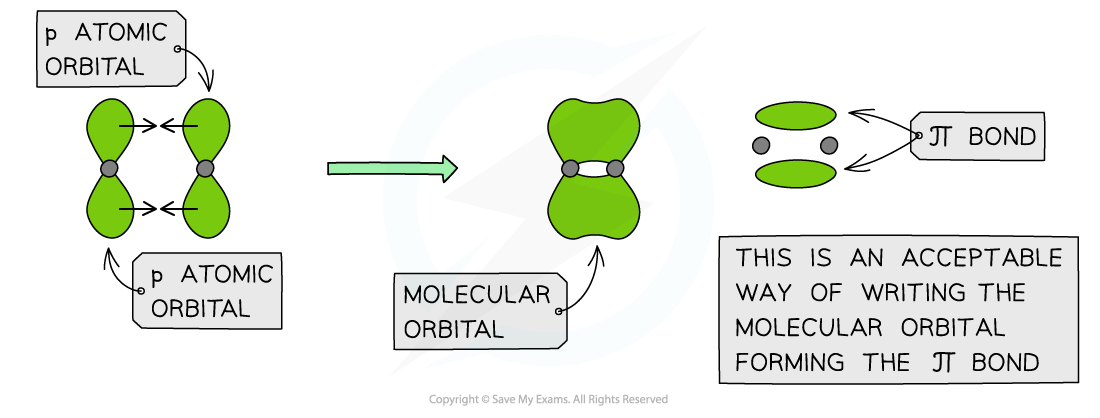 π orbitals can be formed from the end-on overlap of p orbitals
π orbitals can be formed from the end-on overlap of p orbitals
Examples of sigma & pi bonds
- Hydrogen
- The hydrogen atom has only one s orbital
- The s orbitals of the two hydrogen atoms will overlap to form a σ bond

Direct overlap of the 1s orbitals of the hydrogen atoms results in the formation of a σ bond
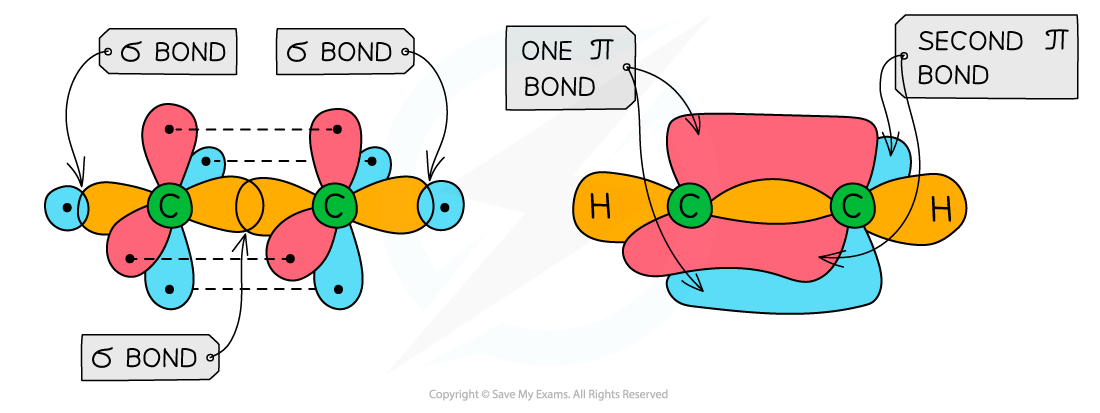
- Ethene
- Each carbon atom uses three of its four electrons to form σ bonds
- Two σ bonds are formed with the hydrogen atoms
- One σ bond is formed with the other carbon atom
- The fourth electron from each carbon atom occupies a p orbital which overlaps sideways with another p orbital on the other carbon atom to form a π bond
- This means that the C-C is a double bond: one σ and one π bond
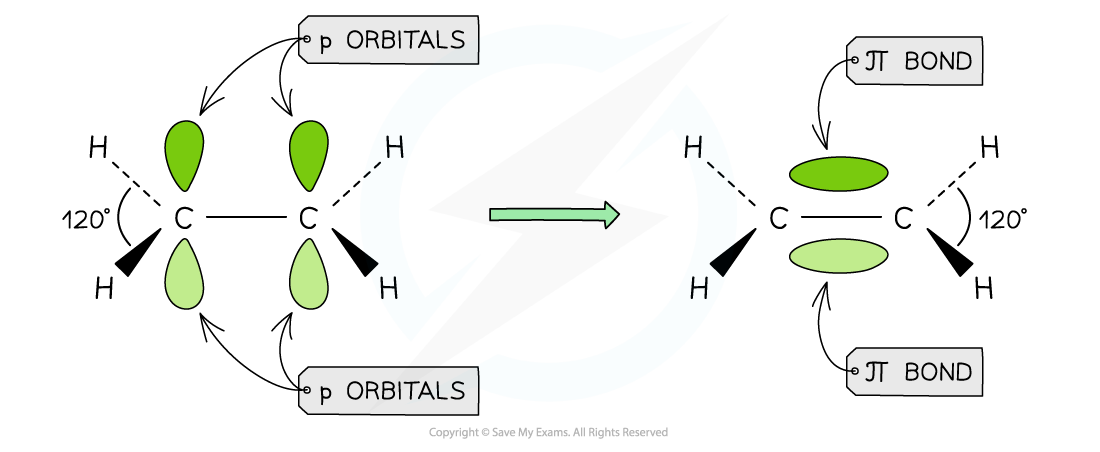
Overlap of the p orbitals results in the forming of a π bond in ethene
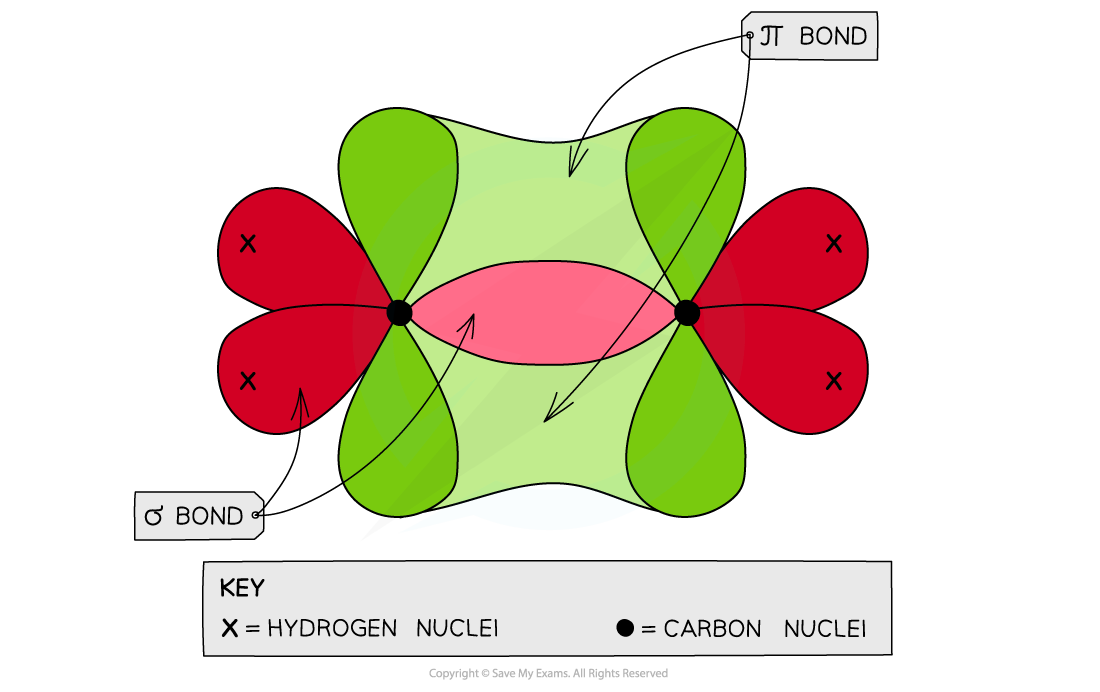
Each carbon atom in ethene forms two sigma bonds with hydrogen atoms and one σ bond with another carbon atom. The fourth electron is used to form a π bond between the two carbon atoms
- Ethyne
- This molecule contains a triple bond formed from two π bonds (at right angles to each other) and one σ bond
- Each carbon atom uses two of its four electrons to form σ bonds
- One σ bond is formed with the hydrogen atom
- One σ bond is formed with the other carbon atom
- Two electrons are used to form two π bonds with the other carbon atom
Ethyne has a triple bond formed from two π bonds and one σ bond between the two carbon atoms
- Hydrogen cyanide
- Hydrogen cyanide contains a triple bond
- One σ bond is formed between the H and C atom (overlap of an sp C hybridised orbital with the 1s H orbital)
- A second σ bond is formed between the C and N atom (overlap of an sp C hybridised orbital with a p orbital of N)
- The remaining two sets of p orbitals of nitrogen and carbon will overlap to form two π bonds at right angles to each other
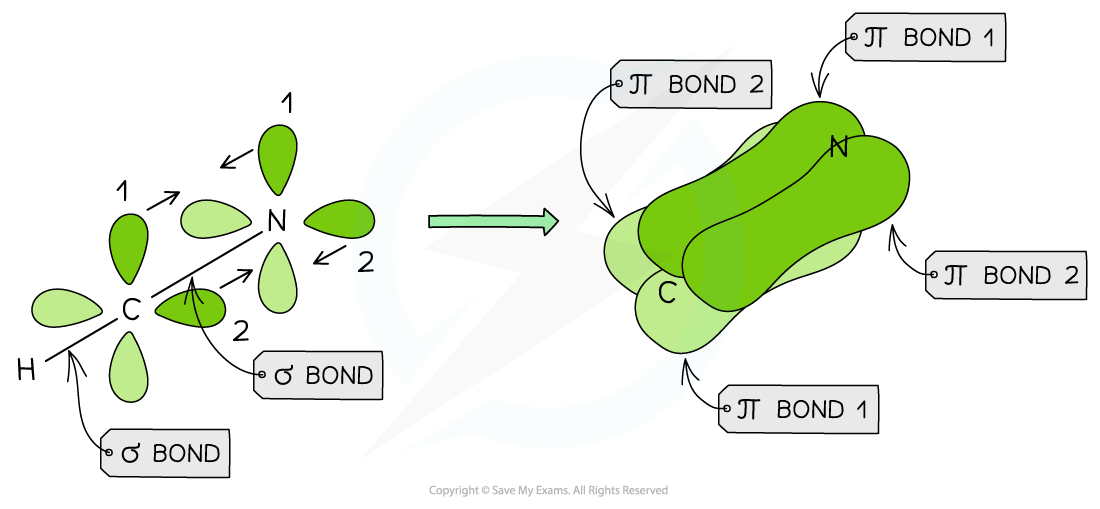 Hydrogen cyanide has a triple bond formed from the overlap of two sets of p orbitals of nitrogen and carbon and the overlap of an sp hybridised carbon orbital and a p orbital on the nitrogen
Hydrogen cyanide has a triple bond formed from the overlap of two sets of p orbitals of nitrogen and carbon and the overlap of an sp hybridised carbon orbital and a p orbital on the nitrogen
- Nitrogen
- Nitrogen too contains a triple bond
- The triple bond is formed from the overlap of the s orbitals on each N to form a σ bond and the overlap of two sets of p orbitals on the nitrogen atoms to form two π bonds
- These π bonds are at right angles to each other
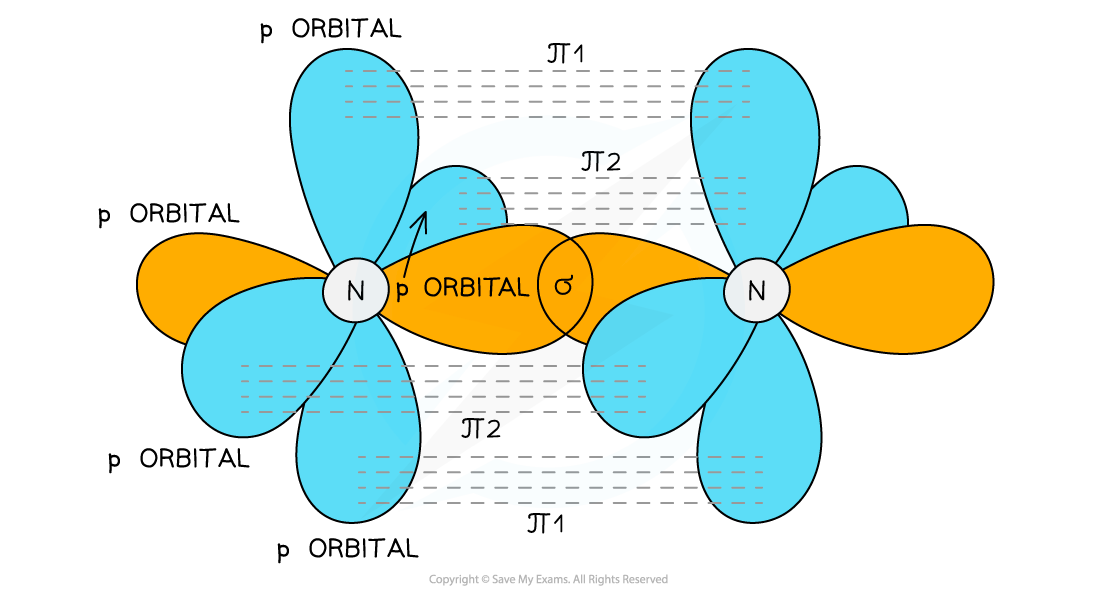
The triple bond is formed from two π bonds and one σ bond
Hybridisation
- The p atomic orbitals can also overlap end-on to form σ bonds
- In order for them to do this, they first need to become modified in order to gain s orbital character
- The orbitals are therefore slightly changed in shape to make one of the p orbital lobes bigger
- This mixing of atomic orbitals to form covalent bonds is called hybridisation
- Mixing one s orbital with three p orbitals is called sp3 hybridisation (each orbital has ¼ s character and ¾ p character)
- Mixing one s orbital with two p orbitals is called sp2 hybridisation
- Mixing one s orbital with one p orbital forms sp hybridised orbitals
π orbitals can be formed from the end-on overlap of p orbitals
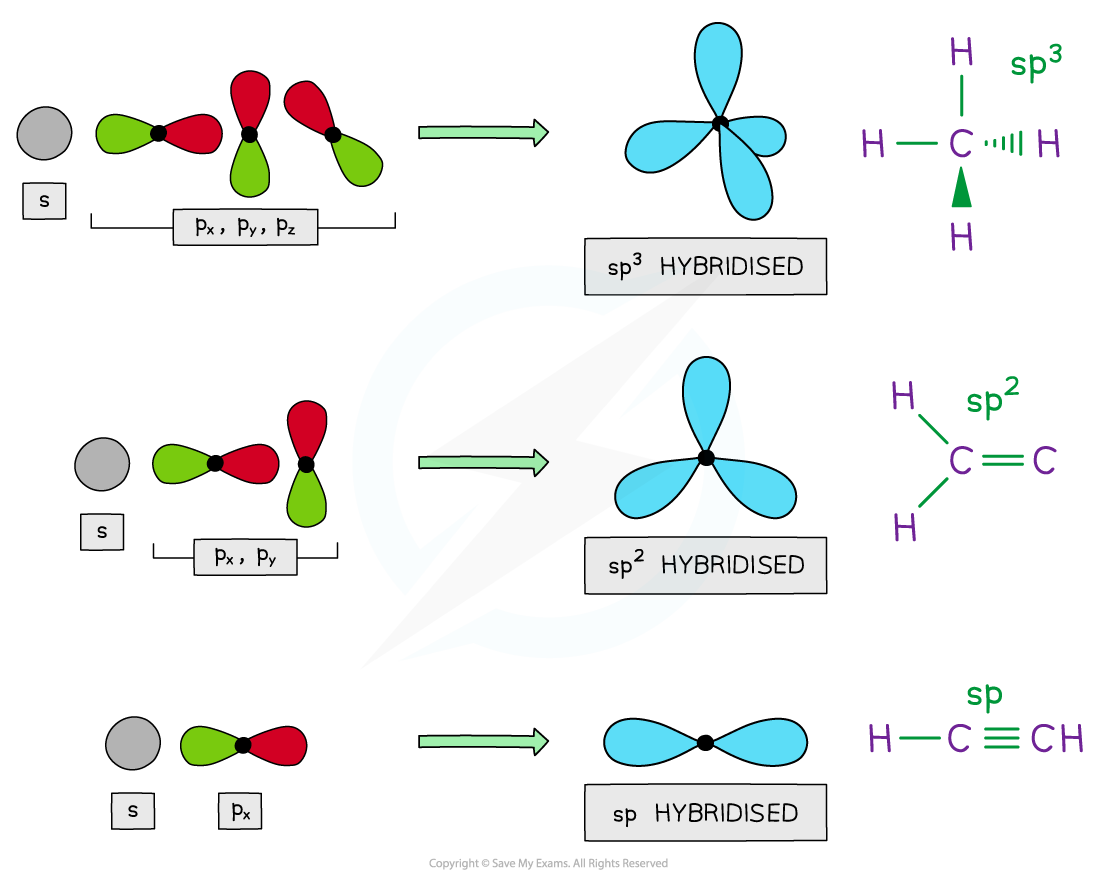
The mixing of s orbitals with p orbitals to form molecular bonds is called hybridisation
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









