- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记1.3.8 Coordinate Bonding
Dative Bonding: Definition & Examples
- In simple covalent bonds the two atoms involved shares electrons
- Some molecules have a lone pair of electrons that can be donated to form a bond with an electron-deficient atom
- An electron-deficient atom is an atom that has an unfilled outer orbital
- So both electrons are from the same atom
- This type of bonding is called dative covalent bonding or coordinate bond
- An example of a dative bond is in an ammonium ion
- The hydrogen ion, H+ is electron-deficient and has space for two electrons in its shell
- The nitrogen atom in ammonia has a lone pair of electrons which it can donate to the hydrogen ion to form a dative covalent bond
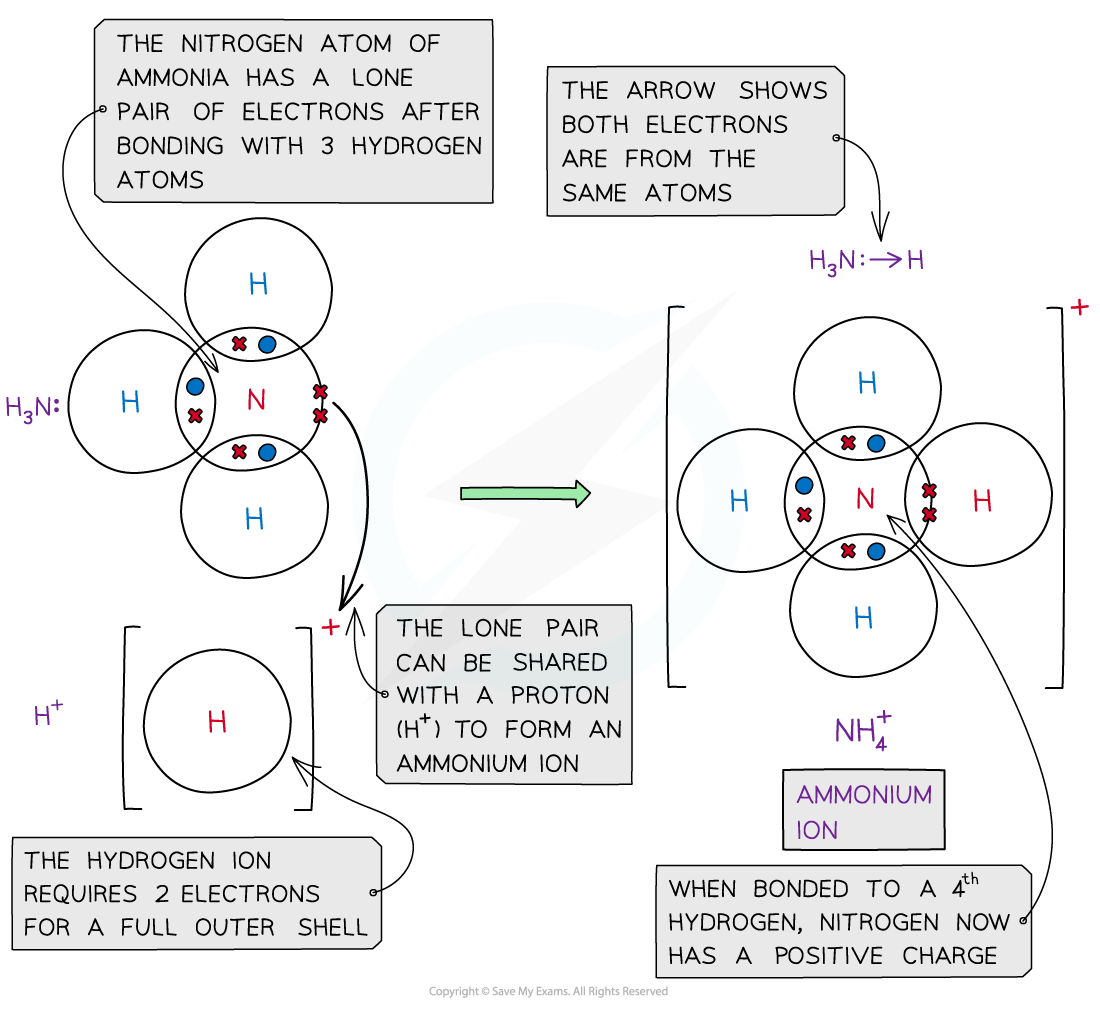
Ammonia (NH3) can donate a lone pair to an electron-deficient proton (H+) to form a charged ammonium ion (NH4+)
- Aluminium chloride is also formed using dative covalent bonding
- At high temperatures aluminium chloride can exist as a monomer (AlCl3)
- The molecule is electron-deficient and needs to electrons complete the aluminium atom’s outer shell
- At lower temperatures the two molecules of AlCl3 join together to form a dimer (Al2Cl6)
- The molecules combine because lone pairs of electrons on two of the chlorine atoms form two coordinate bonds with the aluminium atoms
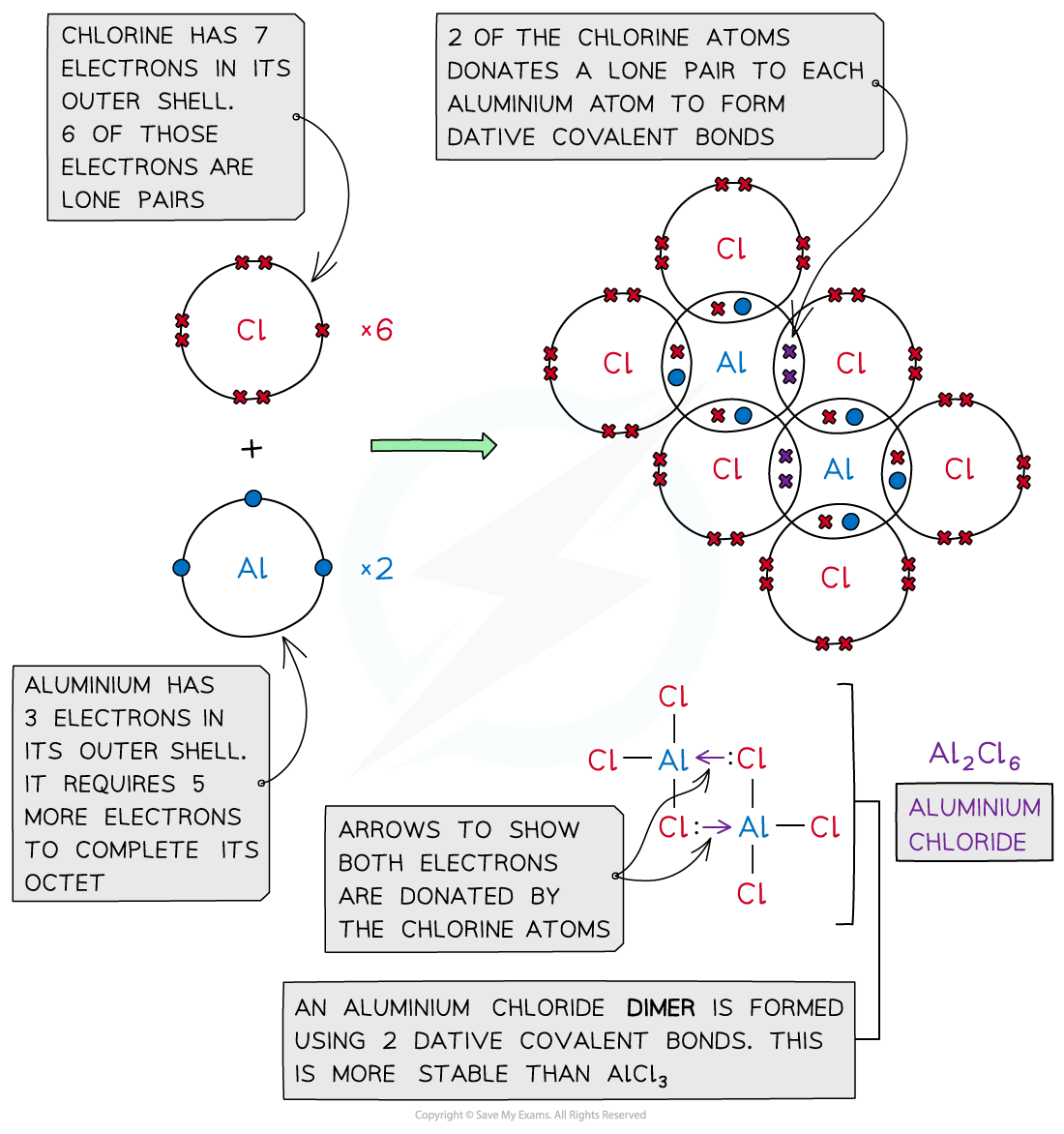
Aluminium chloride is also formed with a dative covalent bond in which two of the chlorine atoms donate their lone pairs to each of the aluminium atoms to form a dimer
Exam Tip
In dative covalent bonding, both electrons in the covalent bond are shared by one atom.A dative covalent bond uses an arrow from the donated pair of electrons to the electron-deficient atom.
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









