- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记1.3.7 Covalent Bonding
Covalent Bonding: Definition & Examples
- Covalent bonding occurs between two nonmetals
- A covalent bond involves the electrostatic attraction between nuclei of two atoms and the bonding electrons of their outer shells
- No electrons are transferred but only shared in this type of bonding
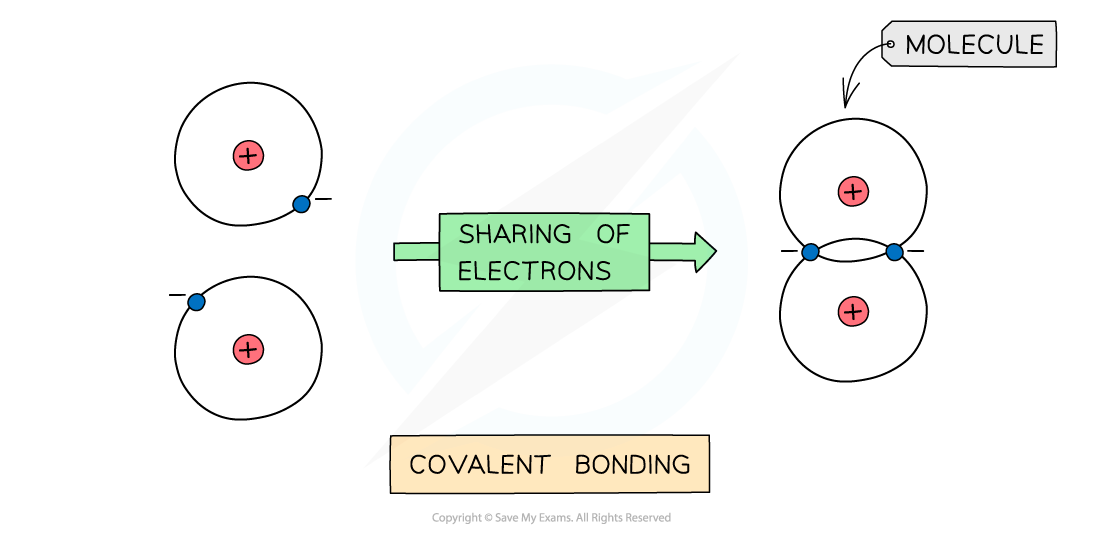
The positive nucleus of each atom has an attraction for the bonding electrons shared in the covalent bond
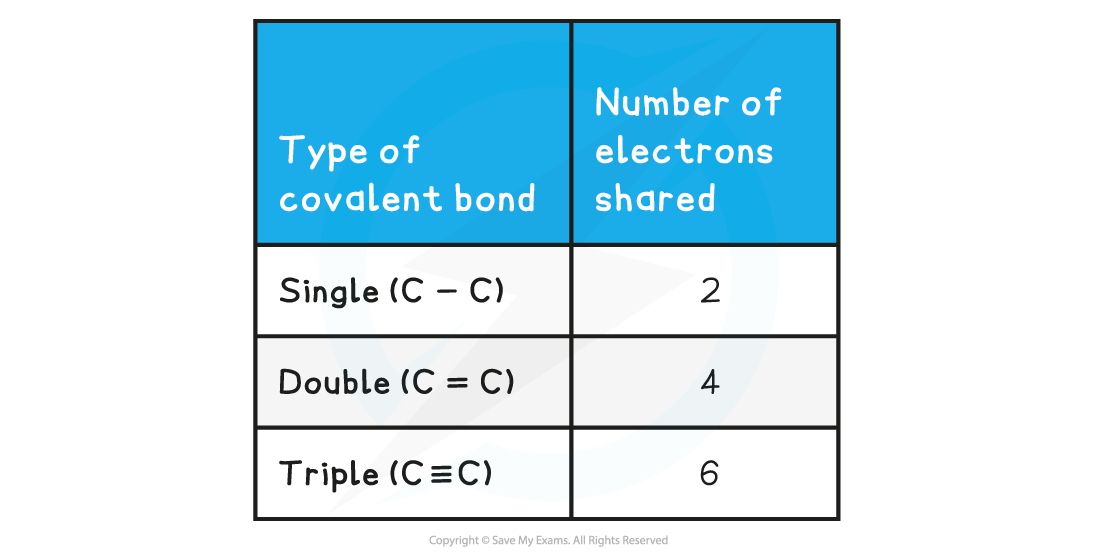
- Non-metals are able to share pairs of electrons to form different types of covalent bonds
- Sharing electrons in the covalent bond allows each of the 2 atoms to achieve an electron configuration similar to a noble gas
- This makes each atom more stable
Covalent bonds & shared electrons table
Dot & cross diagrams
- Dot and cross diagrams are used to represent covalent bonding
- They show just the outer shell of the atoms involved
- To differentiate between the two atoms involved, dots for electrons of one atom and crosses for electrons of the other atom are used
- Electrons are shown in pairs on dot-and-cross diagrams
Single covalent bonding
Hydrogen, H2
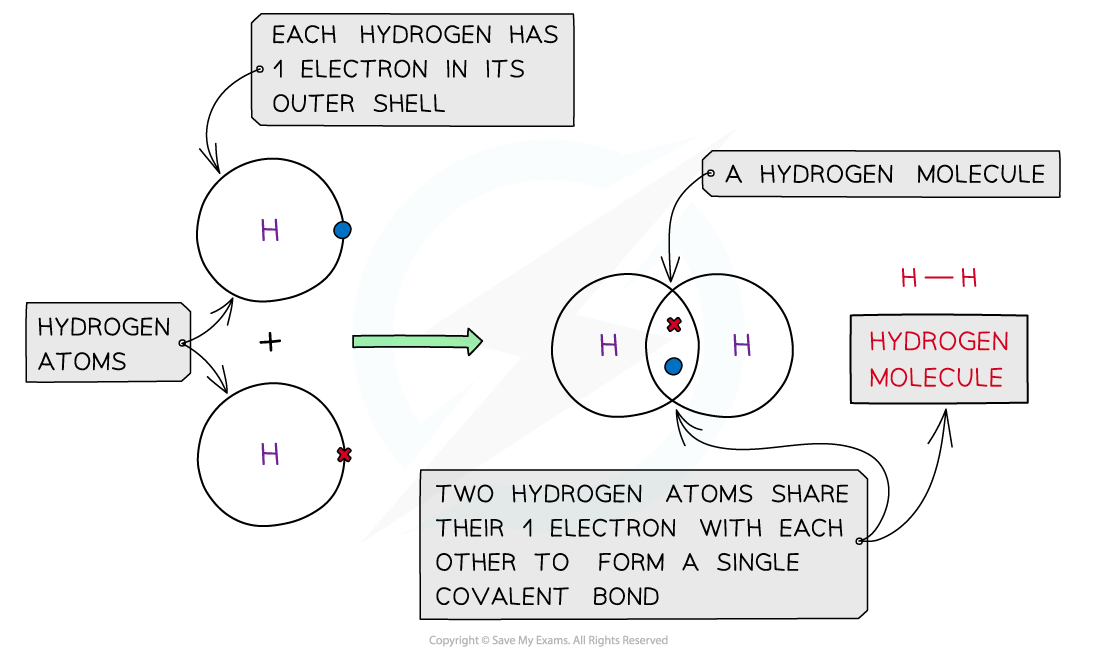 Covalent bonding in hydrogen
Covalent bonding in hydrogen
Chlorine, Cl2
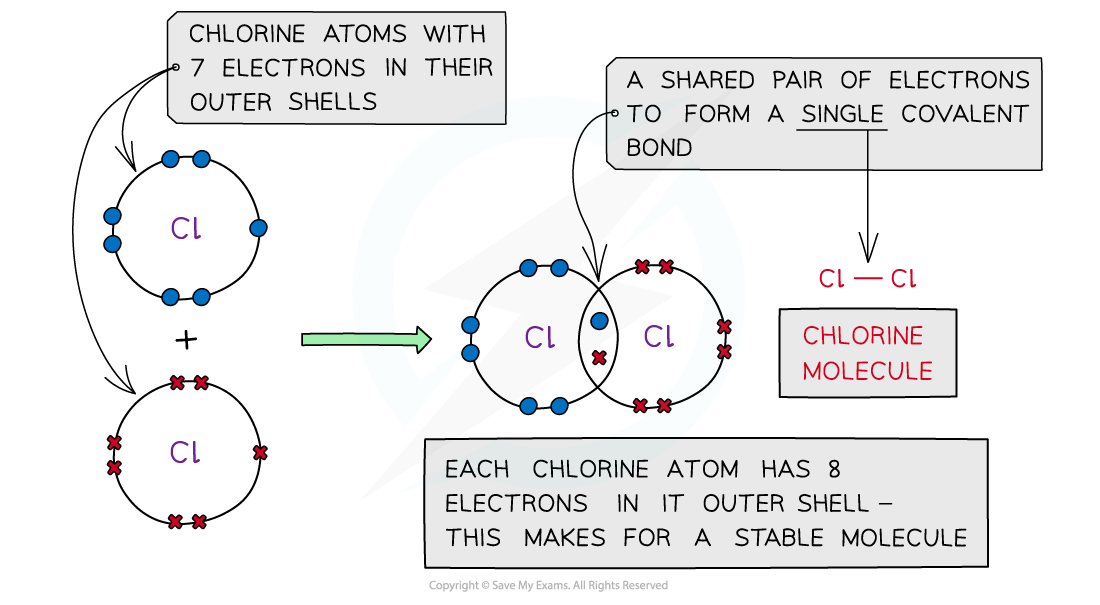
Covalent bonding in chlorine
Hydrogen Chloride, HCl
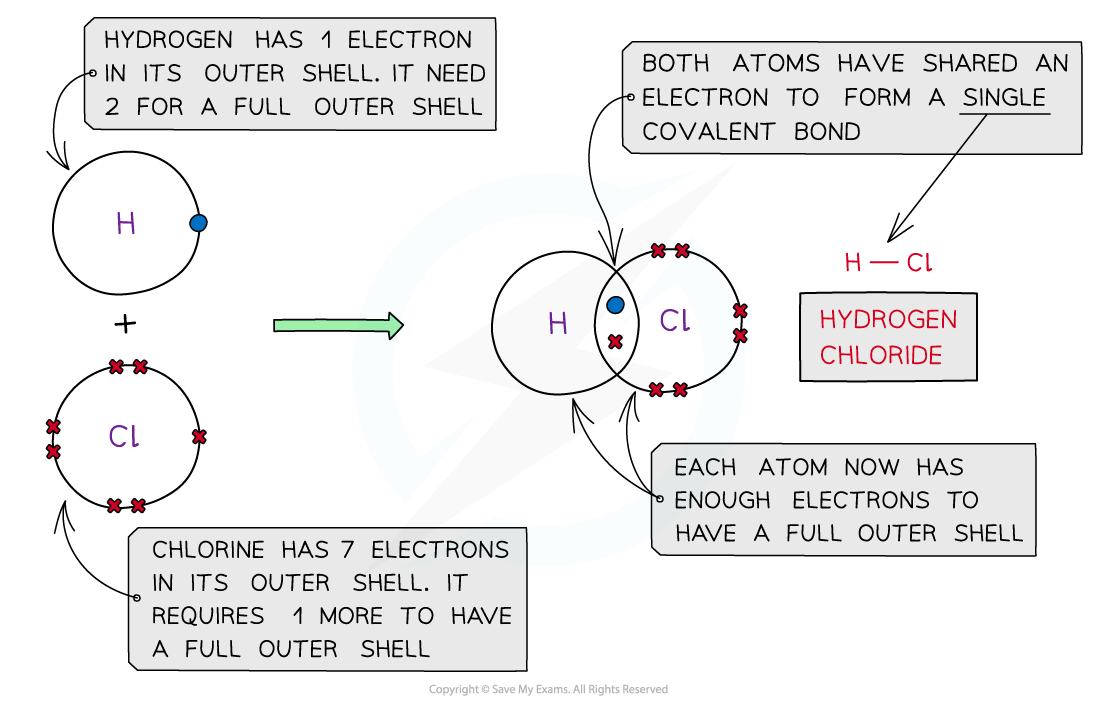
Covalent bonding in hydrogen chloride
Ammonia, NH3
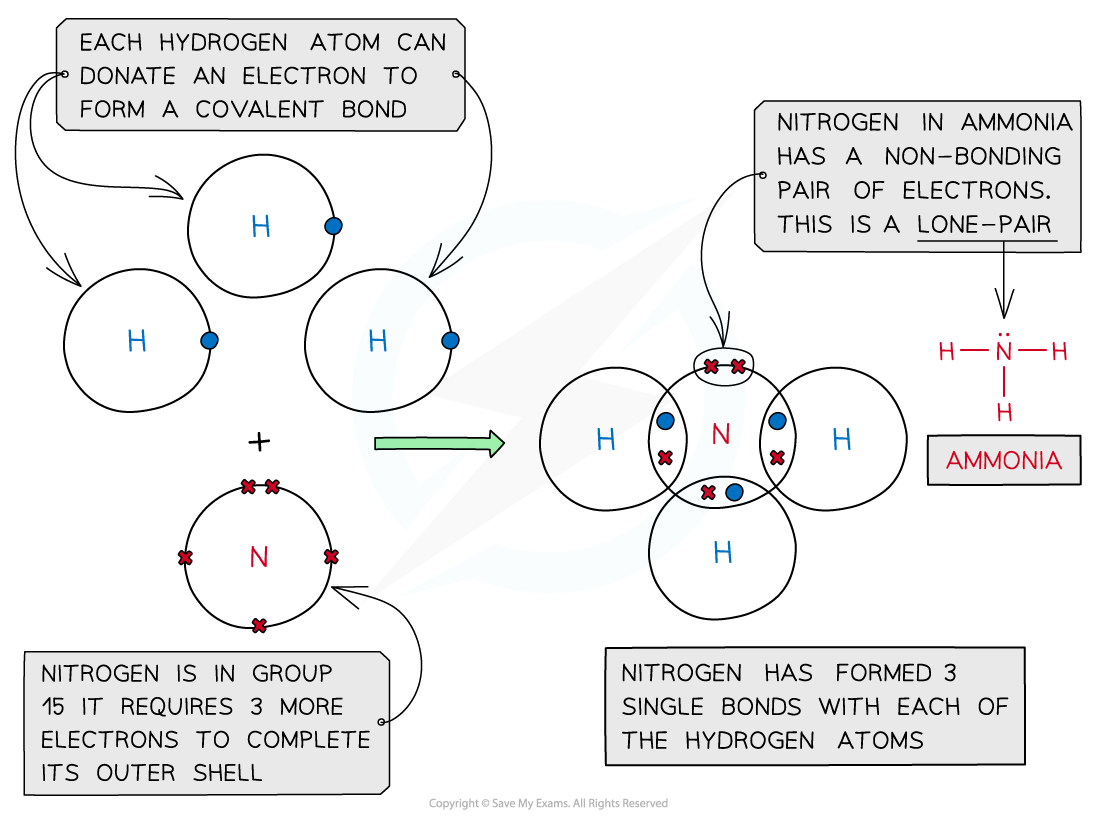
Covalent bonding in ammonia
Methane, CH4
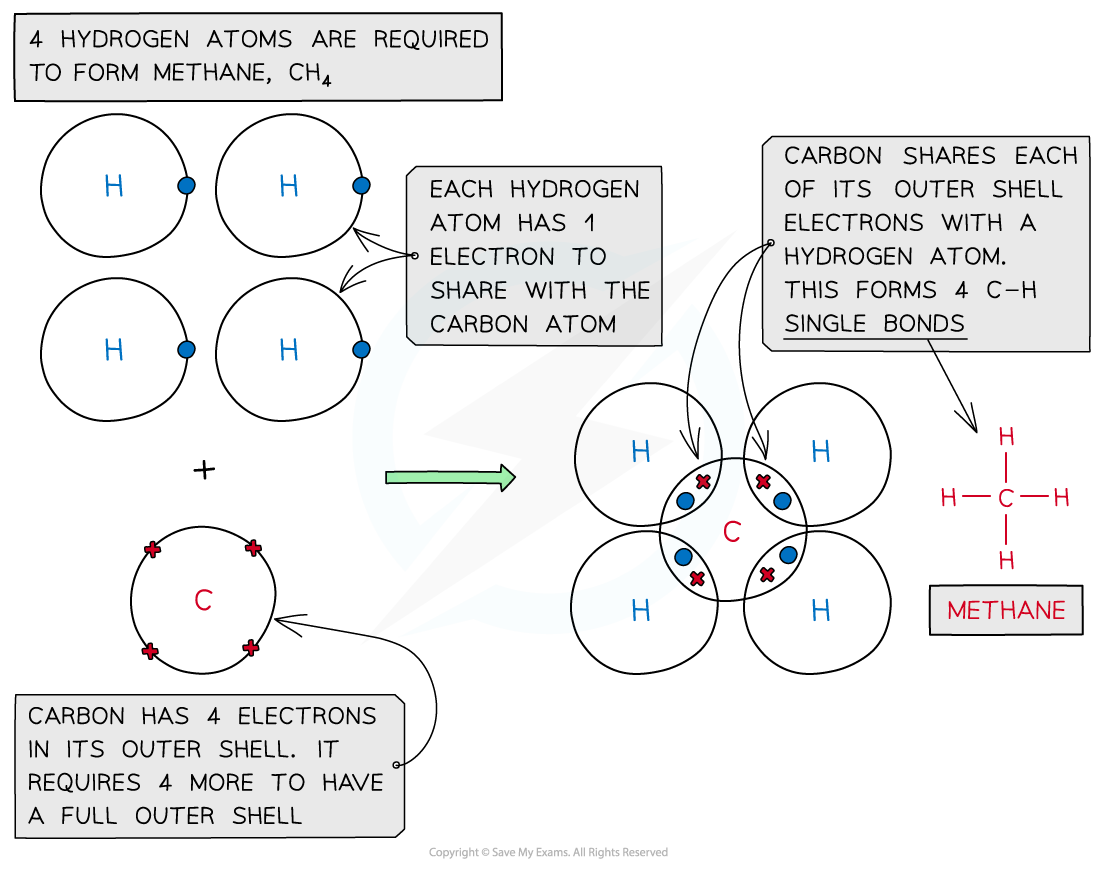
Covalent bonding in methane
Ethane, C2H6
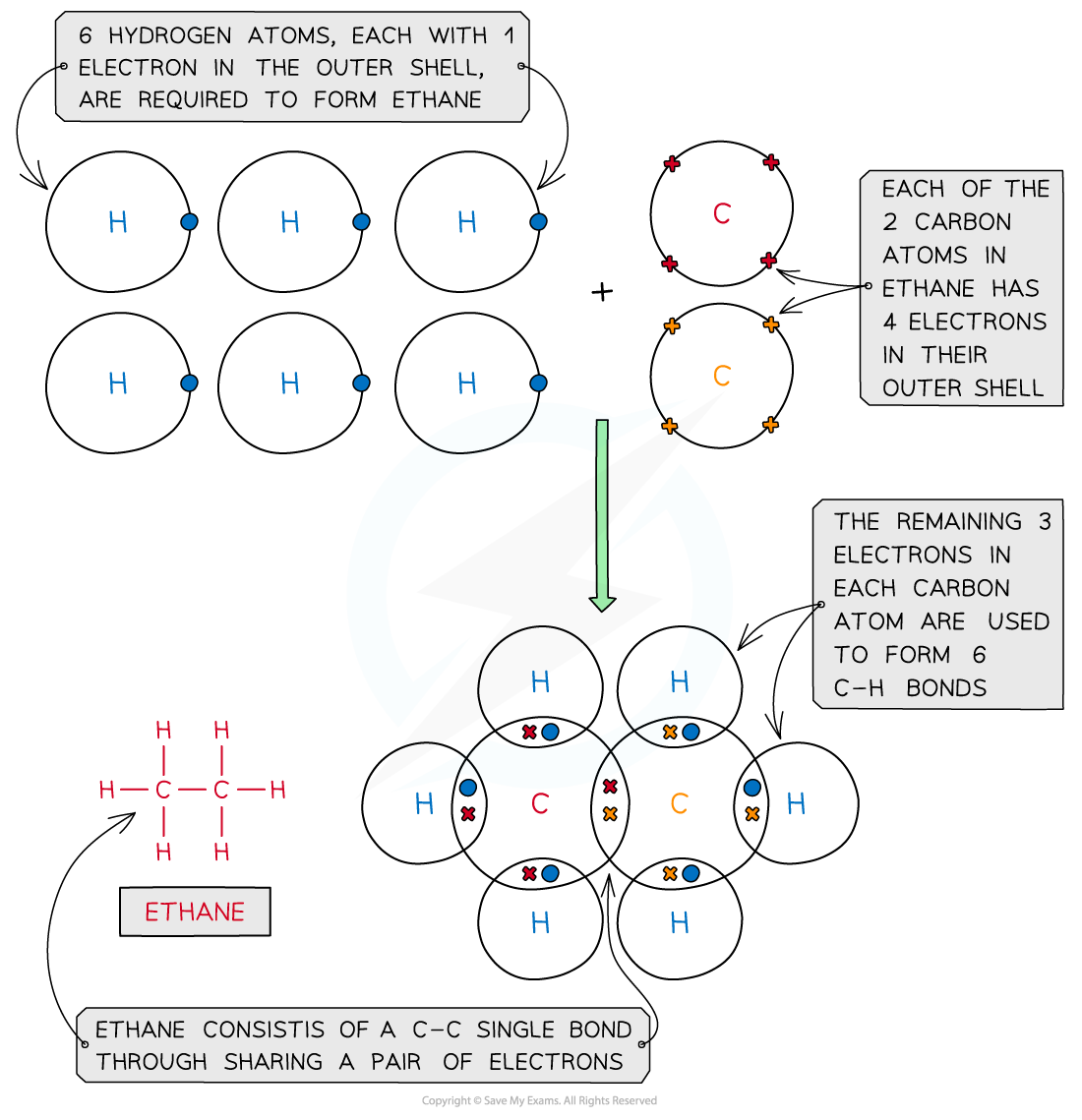
Covalent bonding in ethane
Double covalent bonding
Oxygen, O2
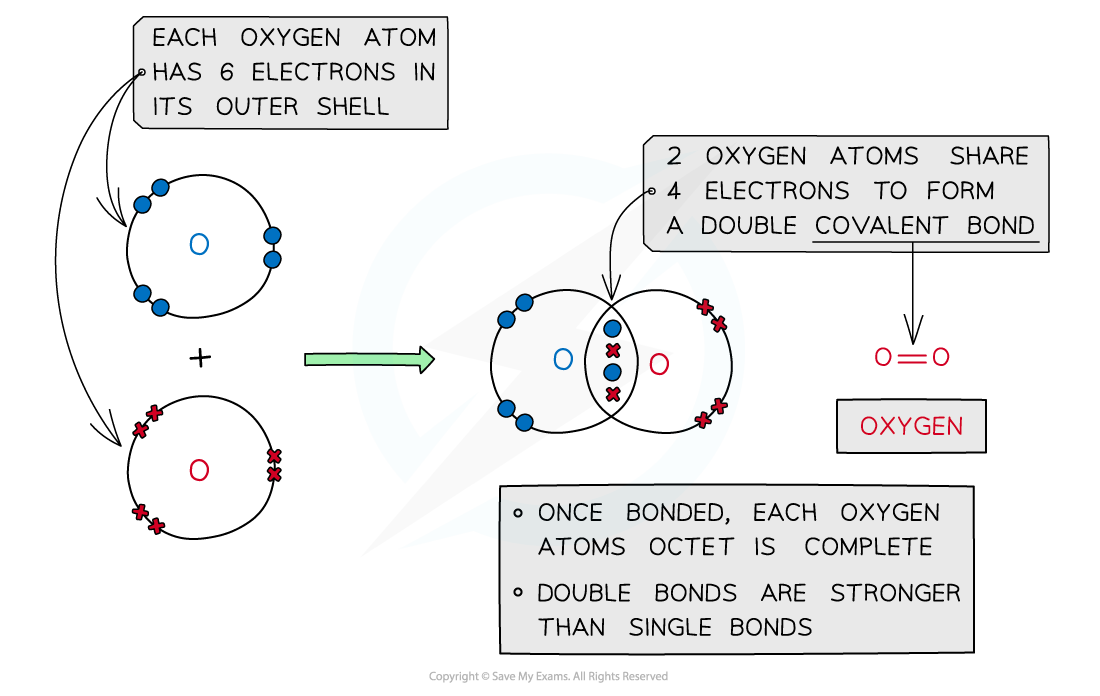 Covalent bonding in oxygen
Covalent bonding in oxygen
Carbon dioxide, CO2
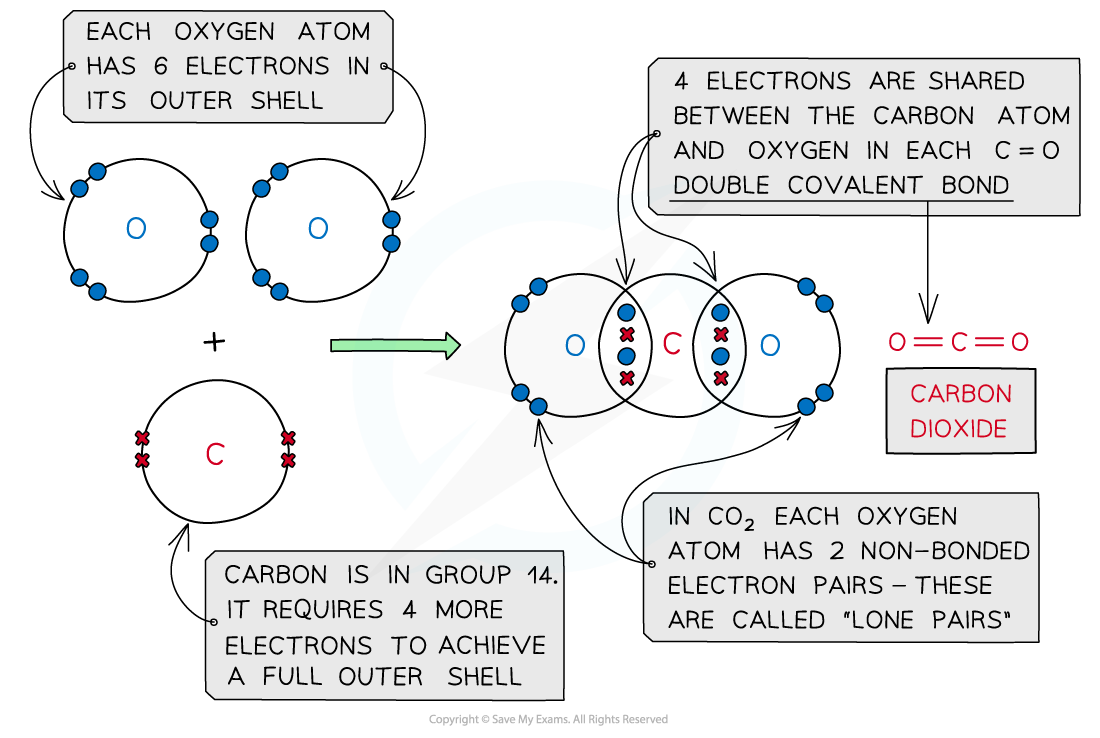
Covalent bonding in carbon dioxide
Ethene, C2H4
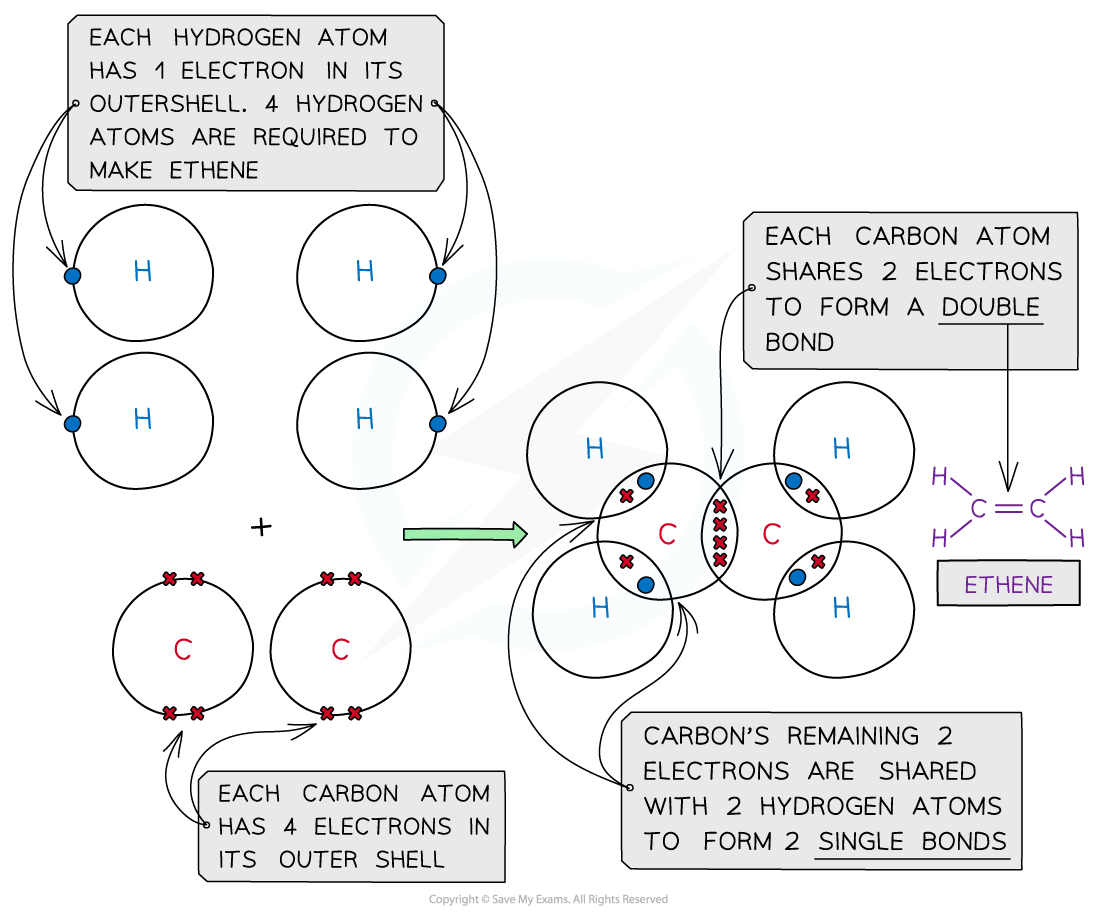
Covalent bonding in ethene
Triple covalent bonding
Nitrogen, N2
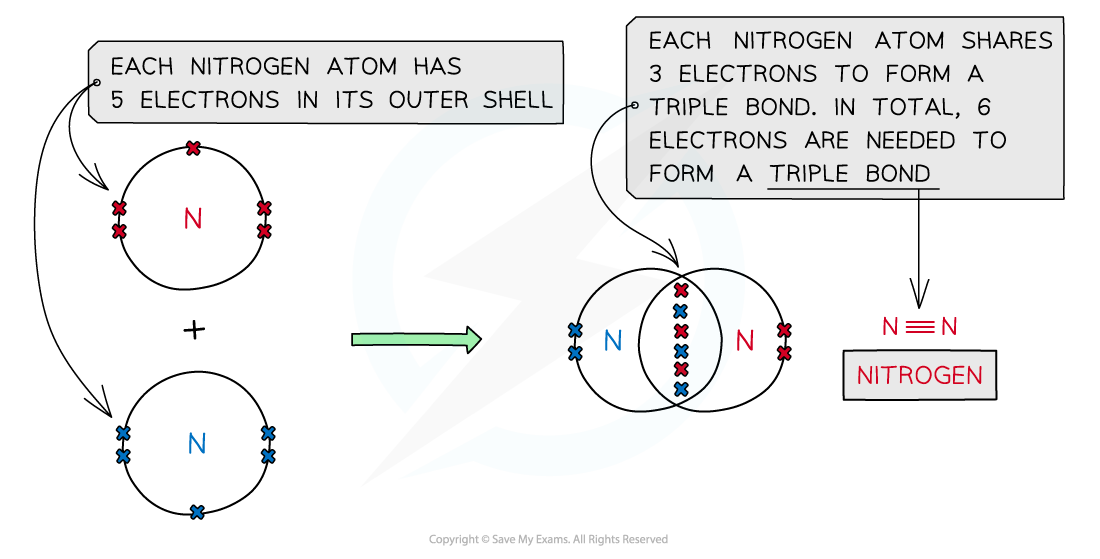 Covalent bonding in nitrogen
Covalent bonding in nitrogen
- In some instances, the central atom of a covalently bonded molecule can accommodate more or less than 8 electrons in its outer shell
- Being able to accommodate more than 8 electrons in the outer shell is known as ‘expanding the octet rule’
- Accommodating less than 8 electrons in the outer shell means than the central atom is ‘electron deficient’
- Some examples of this occurring can be seen with Period 3 elements
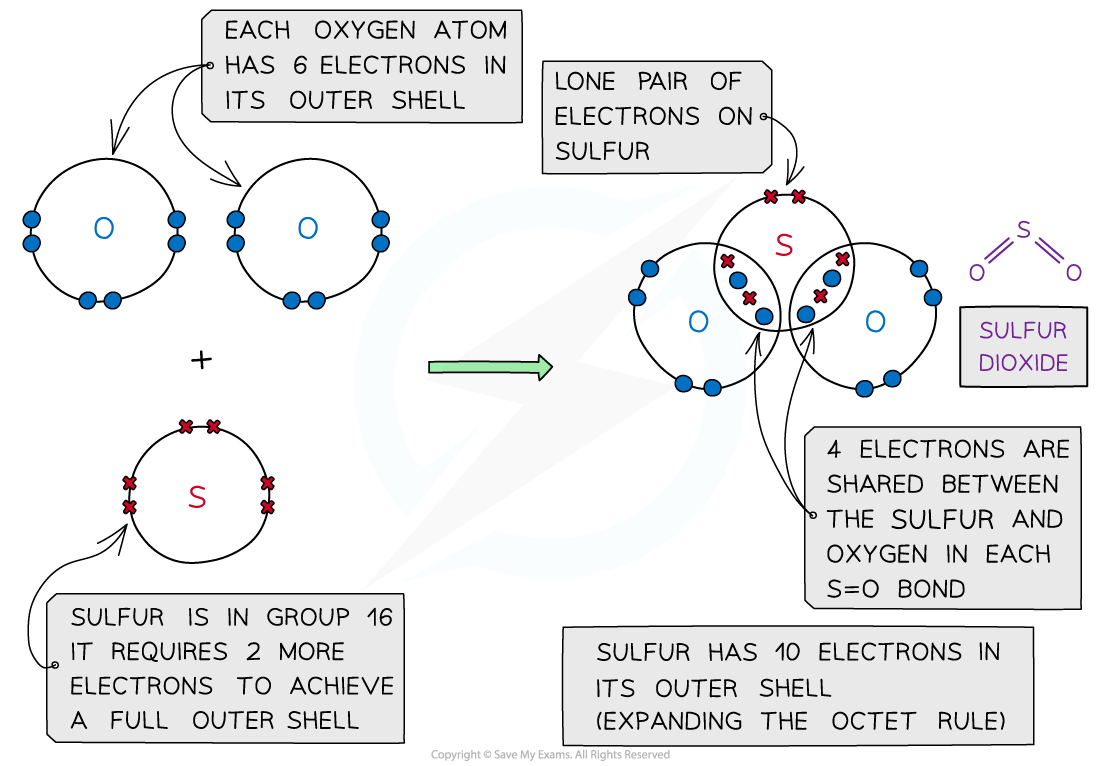
Sulfur dioxide, SO2 – dot and cross diagram
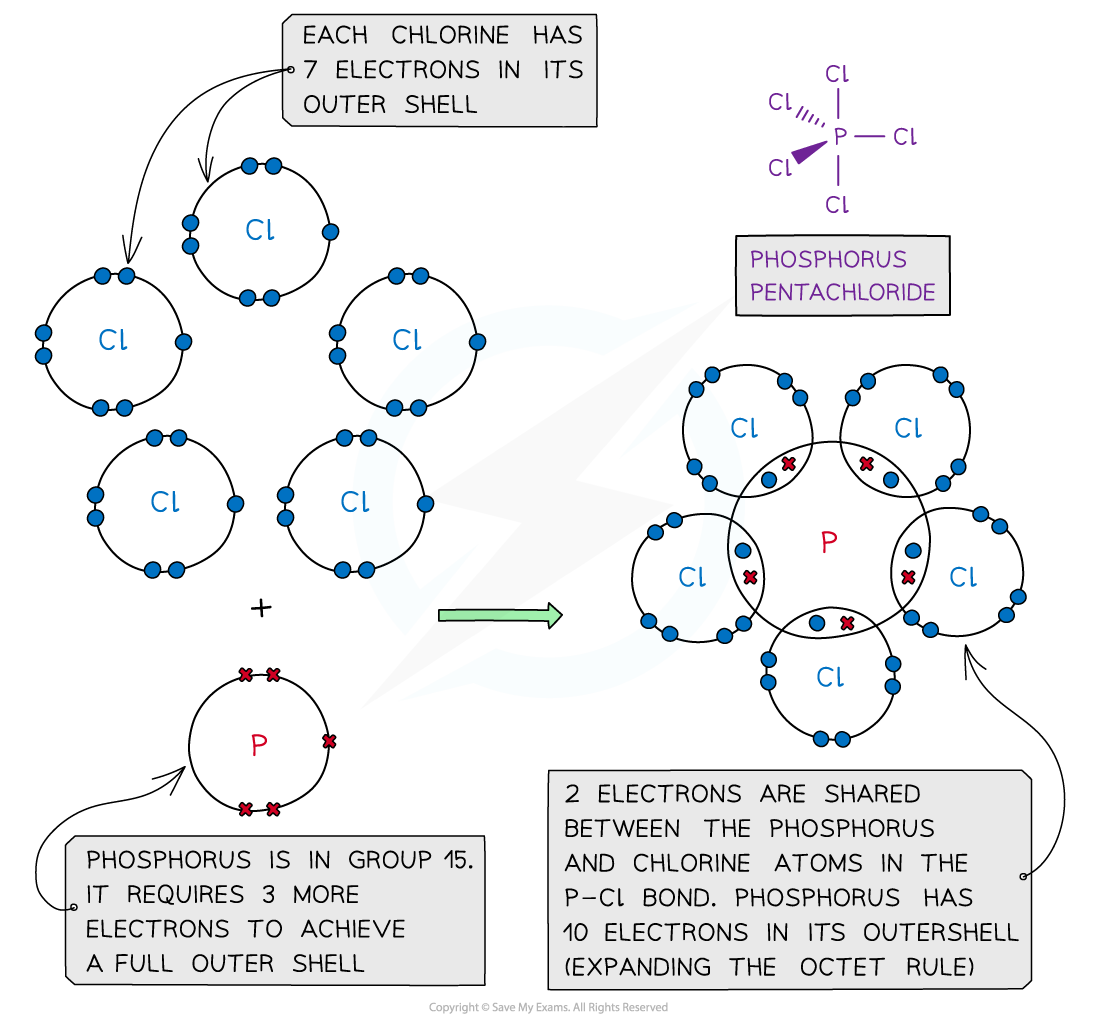
Phosphorus pentachloride, PCl5 – dot and cross diagram
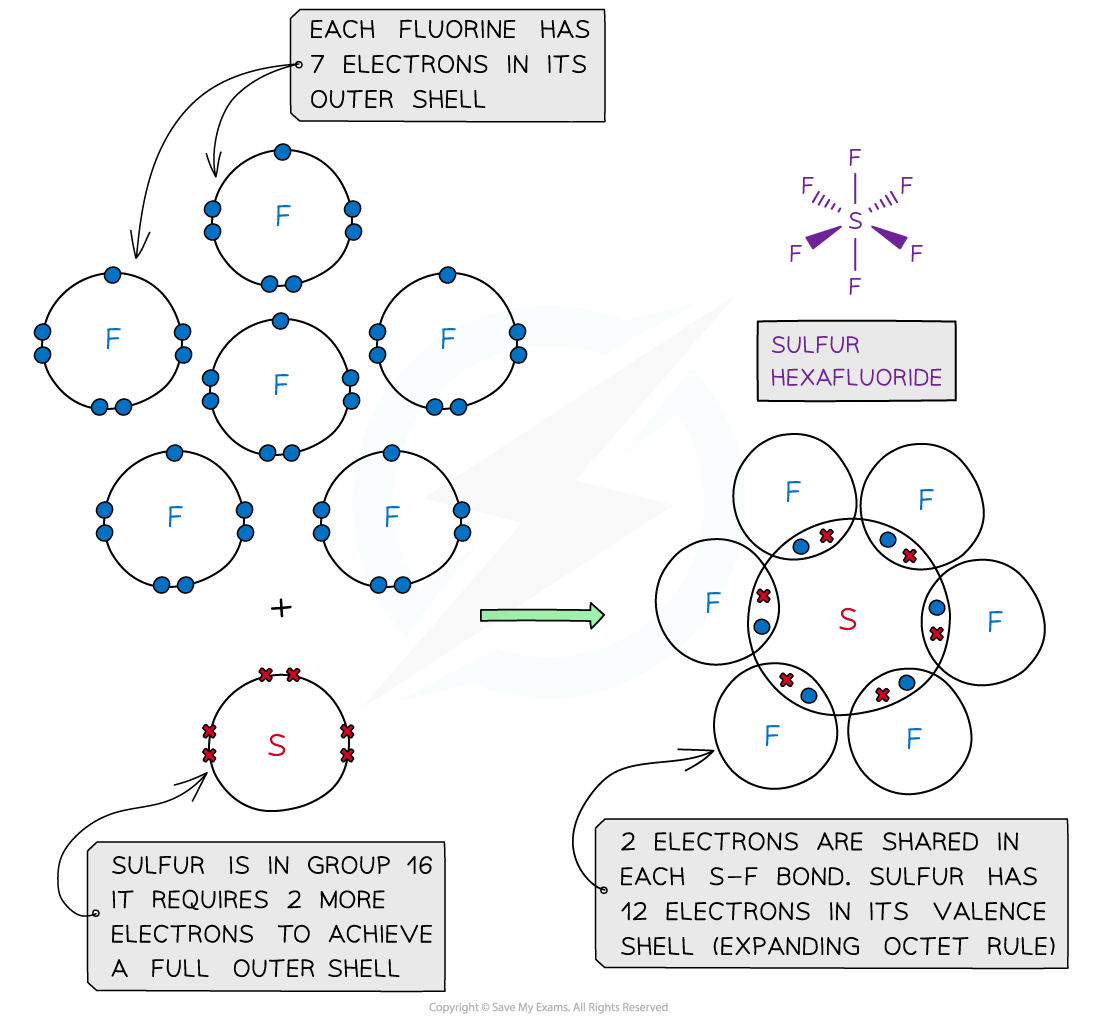
Sulfur hexafluoride, SF6 – dot and cross diagram
Exam Tip
Covalent bonding takes place between nonmetal atoms.Remember to use the Periodic Table to decide how many electrons are in the outer shell of a nonmetal atom.
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








