- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记1.2.5 Empirical & Molecular Formulae
Empirical & Molecular Formulae
- The molecular formula is the formula that shows the number and type of each atom in a molecule
- Eg. the molecular formula of ethanoic acid is C2H4O2
- The empirical formula is the simplest whole number ratio of the elements present in one molecule or formula unit of the compound
- Eg. the empirical formula of ethanoic acid is CH2O
- Organic molecules often have different empirical and molecular formulae
- Simple inorganic molecules however have often similar empirical and molecular formulae
- Ionic compounds always have similar empirical and molecular formulae
Empirical & Molecular Formulae Calculations
Empirical formula
- Empirical formula is the simplest whole number ratio of the elements present in one molecule or formula unit of the compound
- It is calculated from knowledge of the ratio of masses of each element in the compound
- The empirical formula can be found by determining the mass of each element present in a sample of the compound
- It can also be deduced from data that give the percentage compositions by mass of the elements in a compound
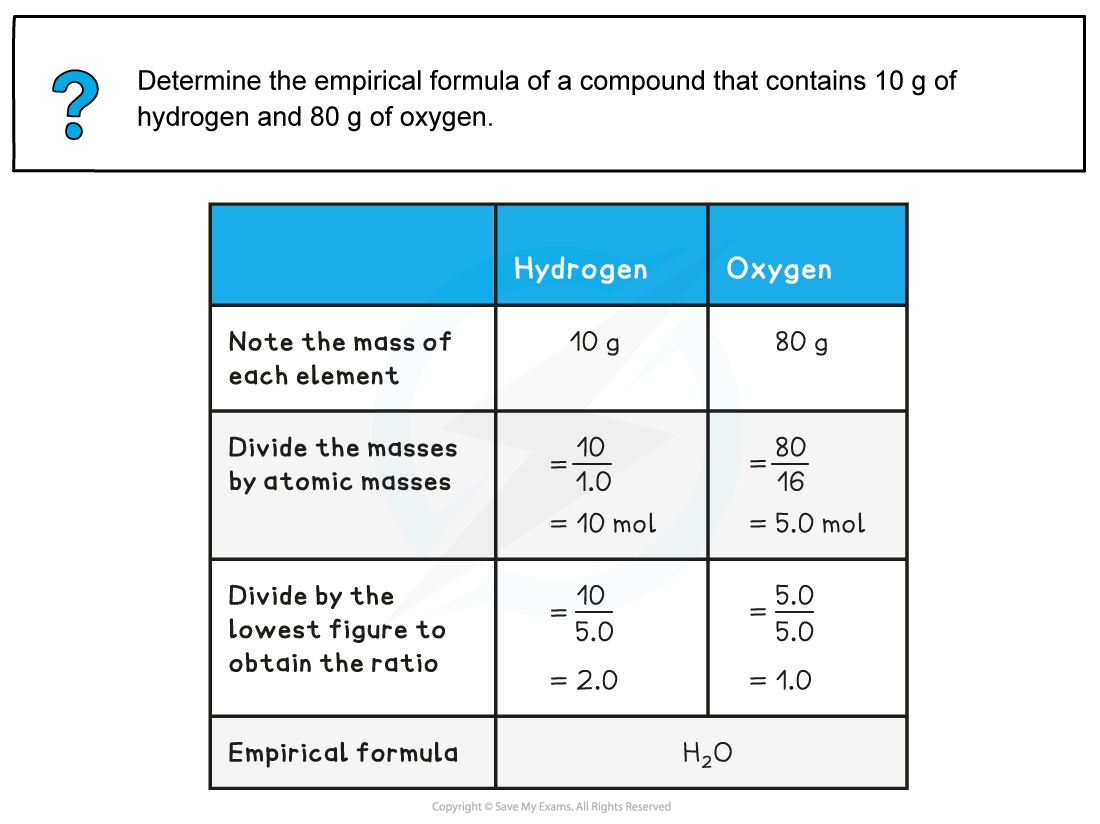
Worked Example: Empirical formula from mass
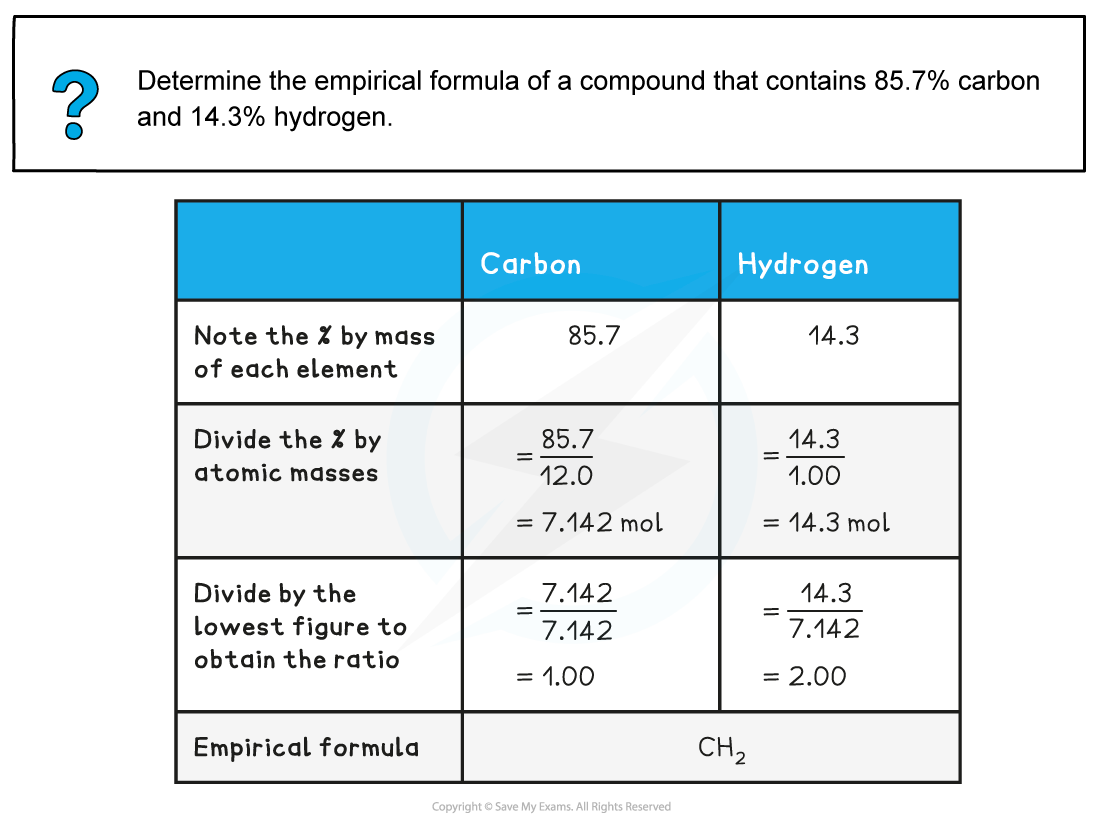
Worked Example 2: Empirical formula from %
Molecular formula
- The molecular formula gives the exact numbers of atoms of each element present in the formula of the compound
- The molecular formula can be found by dividing the relative formula mass of the molecular formula by the relative formula mass of the empirical formula
- Multiply the number of each element present in the empirical formula by this number to find the molecular formula
Worked example: Calculating molecular formula
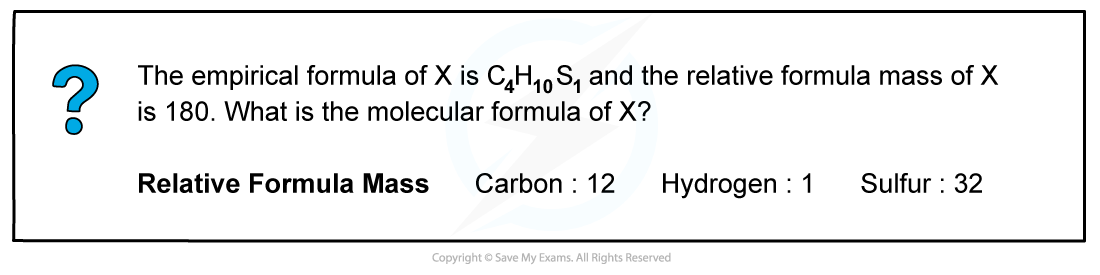
Answer
- Step 1: Calculate relative formula mass of empirical formula
Relative formula mass = (C x 4) + (H x 10) + (S x 1)
Relative formula mass = (12 x 4) + (1 x 10) + (32 x 1)
Relative formula mass = 90
- Step 2: Divide relative formula mass of X by relative formula mass of empirical formula
Ratio between Mr of X and the Mr of the empirical formula = 180/90
Ratio between Mr of X and the Mr of the empirical formula = 2
- Step 3: Multiply each number of elements by 2
(C4 x 2) + (H10 x 2) + (S1 x 2) = (C8) + (H20) + (S2)
Molecular Formula of X is C8H20S2
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








