- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记1.2.2 The Mole & Avogadro Constant
Mole & Avogadro Constant
- The Avogadro constant (NA or L) is the number of particles equivalent to the relative atomic mass or molecular mass of a substance
- The Avogadro constant applies to atoms, molecules, ions and electrons
- The value of NA is 6.02 x 1023 g mol-1
- The mass of a substance with this number of particles is called a mole (mol)
- The mass of a substance containing the same number of fundamental units as there are atoms in exactly 12.00 g of 12C
- One mole of any element is equal to the relative atomic mass of that element in grams
- One mole of carbon, that is if you had 6.02 x 1023 atoms of carbon in your hand, would have a mass of 12 g
- One mole of water would have a mass of (2 x 1 + 16) = 18 g
Worked Example: Moles
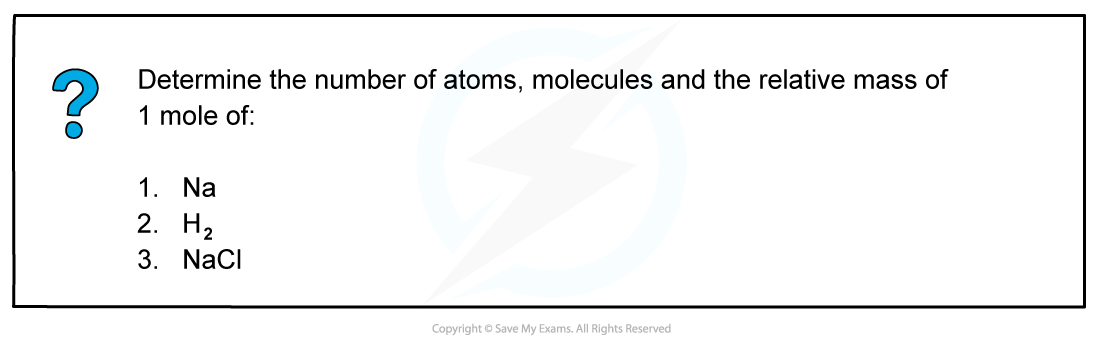
Answer 1
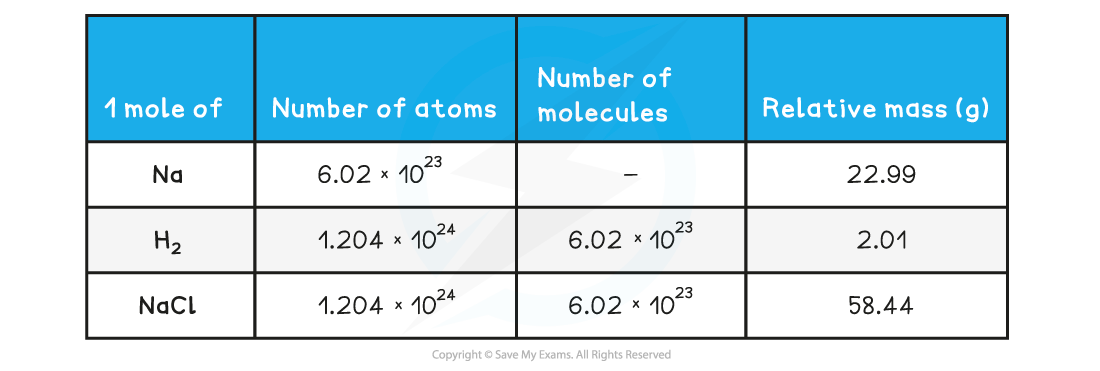
The relative atomic mass of Na is 22.99
Therefore, 1 mol of Na has a mass of 22.99 g mol-1
1 mol of Na will contain 6.02 x 1023 atoms of Na (Avogadro’s constant)
Answer 2
The relative atomic mass of H is 1.005
Since there are 2 H atoms in H2, the mass of 1 mol of H2 is (2 x 1.005) 2.01 g mol-1
1 mol of H2 will contain 6.02 x 1023 molecules of H2
Since there are 2 H atoms in H2, 1 mol of H2 will contain 1.204 x 1024 H atoms
Answer 3
The relative atomic mass of Na and Cl is 22.99 and 35.45 respectively
Therefore, 1 mol of NaCl has a mass of (22.99 + 35.45) 58.44 g mol-1
1 mol of NaCl will contain 6.02 x 1023 molecules of NaCl
Since there are Na and Cl atoms in NaCl, 1 mol of NaCl will contain 1.204 x 1024 atoms in total


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








