- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记1.2.1 Relative Masses
Relative Masses
Atomic Mass Unit
- The mass of a single atom is so small that it is impossible to weigh it directly
- Atomic masses are therefore defined in terms of a standard atom which is called the unified atomic mass unit
- This unified atomic mass is defined as one-twelfth of the mass of a carbon-12 isotope
- The symbol for the unified atomic mass is u (often Da, Dalton, is used as well)
- 1 u = 1.66 x 10-27 kg
Relative atomic mass, Ar
- The relative atomic mass (Ar) of an element is the ratio of the average mass of the atoms of an element to the unified atomic mass unit
- The relative atomic mass is determined by using the average mass of the isotopes of a particular element
- The Ar has no units as it is a ratio and the units cancel each other out
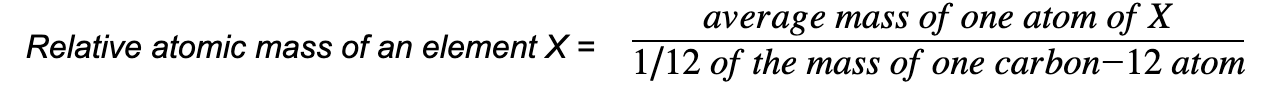
Relative isotopic mass
- The relative isotopic mass is the mass of a particular atom of an isotope compared to the value of the unified atomic mass unit
- Atoms of the same element with a different number of neutrons are called isotopes
- Isotopes are represented by writing the mass number as 20Ne, or neon-20 or Ne-20
- To calculate the average atomic mass of an element the percentage abundance is taken into account
- Multiply the atomic mass by the percentage abundance for each isotope and add them all together
- Divide by 100 to get average relative atomic mass
- This is known as the weighted average of the masses of the isotopes

Relative molecular mass, Mr
- The relative molecular mass (Mr) is the ratio of weighted average mass of a molecule of a molecular compound to the unified atomic mass unit
- The Mr has no units
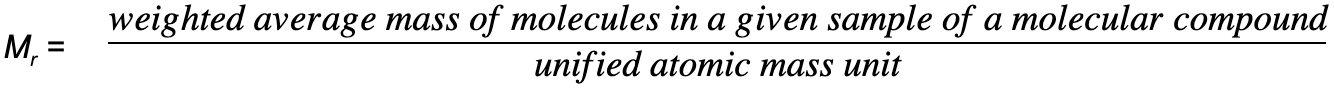
- The Mr can be found by adding up the relative atomic masses of all atoms present in one molecule
- When calculating the Mr the simplest formula for the compound is used, also known as the formula unit
- Eg. silicon dioxide has a giant covalent structure, however the simplest formula (the formula unit) is SiO2
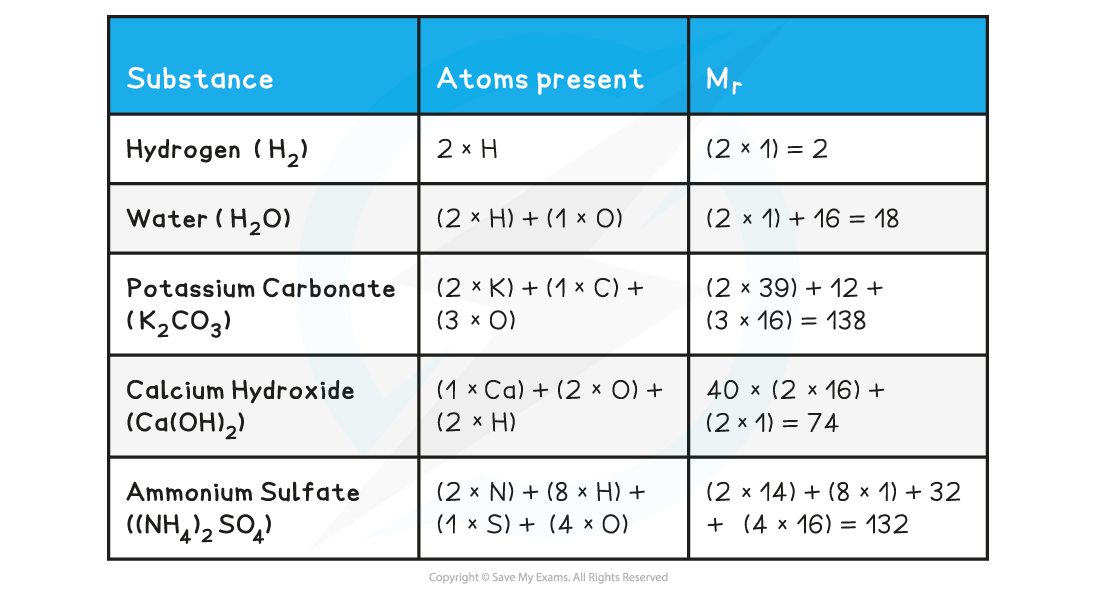
Relative formula mass, Mr
- The relative formula mass (Mr) is used for compounds containing ions
- It has the same units and is calculated in the same way as the relative molecular mass
- In the table above, the Mr for potassium carbonate, calcium hydroxide and ammonium sulfates are relative formula masses
转载自savemyxams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








