- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记1.1.9 Determining Electronic Configurations
Determining Electronic Configurations
- Writing out the electronic configuration tells us how the electrons in an atom or ion are arranged in their shells, subshells and orbitals
- This can be done using the full electron configuration or the shorthand version
- The full electron configuration describes the arrangement of all electrons from the 1s subshell up
- The shorthand electron configuration includes using the symbol of the nearest preceding noble gas to account for however many electrons are in that noble gas
- Ions are formed when atoms lose or gain electrons
- Negative ions are formed by adding electrons to the outer subshell
- Positive ions are formed by removing electrons from the outer subshell
- The transition metals fill the 4s subshell before the 3d subshell but lose electrons from the 4s first and not from the 3d subshell (the 4s subshell is lower in energy)
- The Periodic Table is split up into four main blocks depending on their electronic configuration:
- s block elements
- Have their valence electron(s) in an s orbital
- p block elements
- Have their valence electron(s) in a p orbital
- d block elements
- Have their valence electron(s) in a d orbital
- f block elements
- Have their valence electron(s) in an f orbital
- s block elements
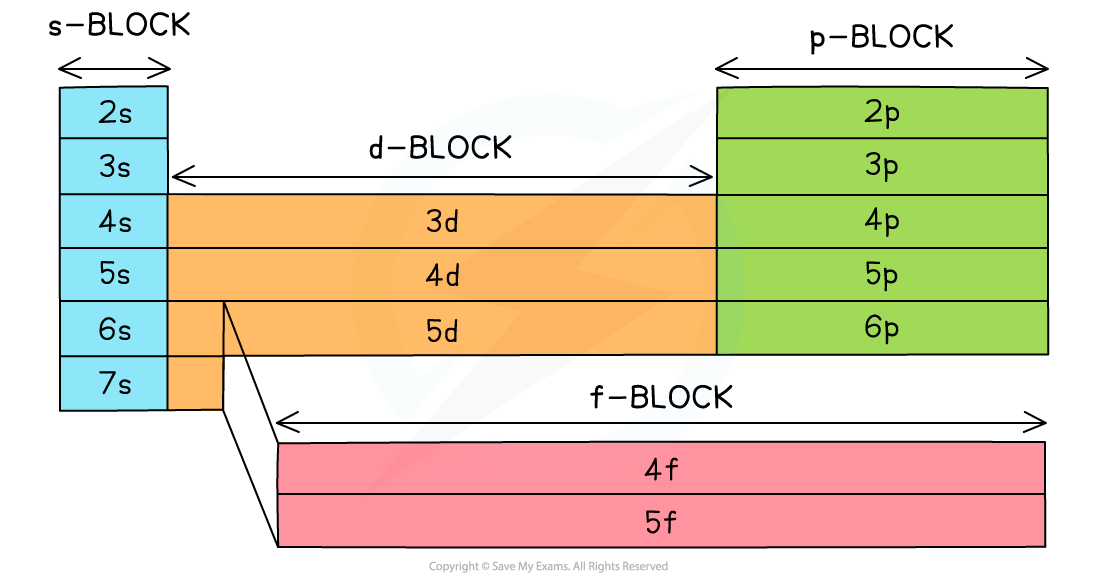
The elements can be divided into four blocks according to their outer shell electron configuration
Exceptions
- Chromium and copper have the following electron configurations, which are different to what you may expect:
- Cr is [Ar] 3d5 4s1 not [Ar] 3d4 4s2
- Cu is [Ar] 3d10 4s1 not [Ar] 3d9 4s2
- This is because the [Ar] 3d5 4s1 and [Ar] 3d10 4s1 configurations are energetically stable
Worked example: Electron configuration
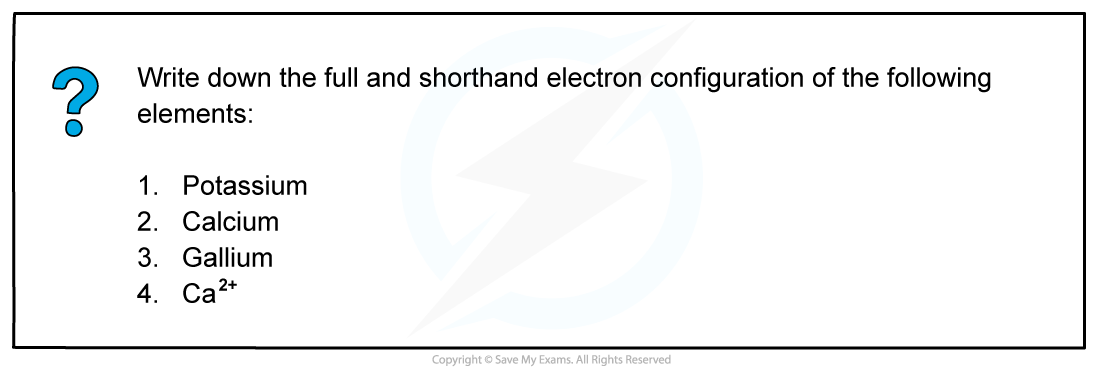
Answer
Answer 1: Potassium has 19 electrons so the full electronic configuration is:
1s2 2s2 2p6 3s2 3p6 4s1
The 4s orbital is lower in energy than the 3d subshell and is therefore filled first
The nearest preceding noble gas to potassium is argon which accounts for 18 electrons so the shorthand electron configuration is:
[Ar] 4s1
Answer 2: Calcium has 20 electrons so the full electronic configuration is:
1s2 2s2 2p6 3s23p6 4s2
The 4s orbital is lower in energy than the 3d subshell and is therefore filled first
The shorthand version is [Ar] 4s2 since argon is the nearest preceding noble gas to calcium which accounts for 18 electrons
Answer 3: Gallium has 31 electrons so the full electronic configuration is:
[Ar] 3d10 4s2 4p1
Answer 4: What this means is that if you ionise calcium and remove two of its outer electrons, the electronic configuration of the Ca2+ ion is identical to that of argon
Ca2+ is 1s22s2 2p6 3s2 3p6
Ar is also 1s2 2s2 2p6 3s23p6
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









