- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记1.1.8 Electron Configuration
Electron Configurations: Basics
- The electron configuration gives information about the number of electrons in each shell, subshell and orbital of an atom
- The subshells are filled in order of increasing energy

The electron configuration shows the number of electrons occupying a subshell in a specific shell
Electron Configurations: Explained
- Electrons can be imagined as small spinning charges which rotate around their own axis in either a clockwise or anticlockwise direction
- The spin of the electron is represented by its direction
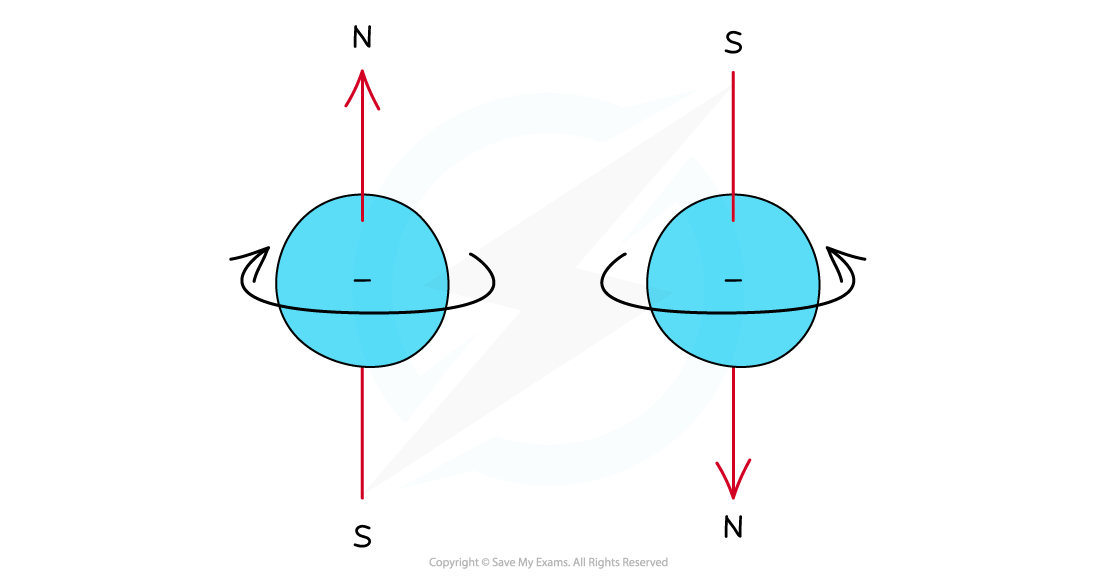
Electrons can spin either in a clockwise or anticlockwise direction around their own axis
- Electrons with similar spin repel each other which is also called spin-pair repulsion
- Electrons will therefore occupy separate orbitals in the same subshell to minimize this repulsion and have their spin in the same direction
- Eg. if there are three electrons in a p subshell, one electron will go into each px, py and pz orbital
 Electron configuration: three electrons in a p subshell
Electron configuration: three electrons in a p subshell
- Electrons are only paired when there are no more empty orbitals available within a subshell in which case the spins are the opposite spins to minimize repulsion
- Eg. if there are four electrons in a p subshell, one p orbital contains 2 electrons with opposite spin and two orbitals contain one electron only

Electron configuration: four electrons in a p subshell
- The principal quantum number indicates the energy level of a particular shell but also indicates the energy of the electrons in that shell
- A 2p electron is in the second shell and therefore has an energy corresponding to n = 2
- Even though there is repulsion between negatively charged electrons (inter-electrons repulsion), they occupy the same region of space in orbitals
- This is because the energy required to jump to successive empty orbital is greater than the inter-electron repulsion
- For this reason, they pair up and occupy the lower energy levels first
Electron Box Notation
- The electron configuration can also be represented using the electrons in boxes notation
- Each box represents an atomic orbital
- The boxes are arranged in order of increasing energy from lowest to highest
- The electrons are represented by opposite arrows to show the spin of the electrons
- Eg. the box notation for titanium is shown below
- Note that since the 3d subshell cannot be either full or half full, the second 4s electron is not promoted to the 3d level and stays in the 4s orbital

The electrons in Titanium are arranged in their orbitals as shown. Electrons occupy the lowest energy levels first before filling those with higher energy
Free Radicals
- A free radical is a species with one or more unpaired electron
- The unpaired electron in the free radical is shown as a dot
- Eg. a chlorine free radical has the electron configuration 1s22s22p63s23p5
- Two of the three p orbitals have paired electrons whereas one of them has an unpaired electron
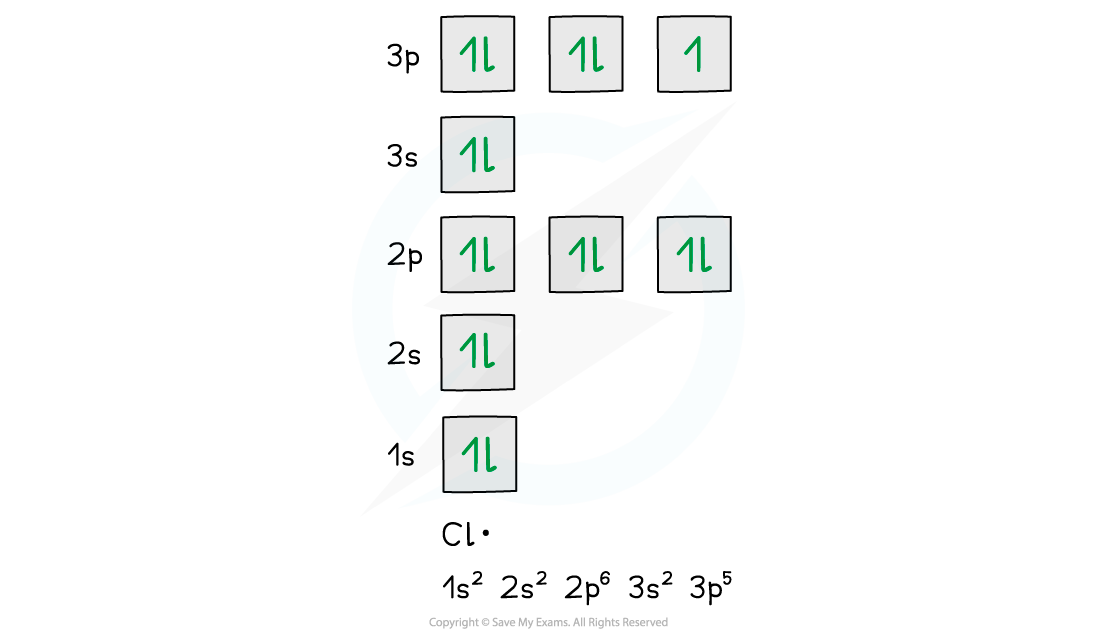
One of the p orbitals has unpaired electrons in a chlorine radical
Exam Tip
Free radicals are formed when a molecule undergoes homolytic fission where the two electrons of a covalent bond are split evenly between the two atoms.
转载自savemyexams


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








