- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记1.1.3 Determining Subatomic Structure
Determining the Subatomic Structure of Atoms & Ions
- An atom is neutral and has no overall charge
- Ions on the other hand are formed when atoms either gain or lose electrons, causing them to become charged
- The number of subatomic particles in atoms and ions can be determined given their atomic (proton) number, mass (nucleon) number and charge
Protons
- The atomic number of an atom and ion determines which element it is
- Therefore, all atoms and ions of the same element have the same number of protons (atomic number) in the nucleus
- E.g. lithium has an atomic number of 3 (three protons) whereas beryllium has atomic number of 4 (4 protons)
- The number of protons equals the atomic (proton) number
- The number of protons of an unknown element can be calculated by using its mass number and number of neutrons:
Mass number = number of protons + number of neutrons
Number of protons = mass number - number of neutrons
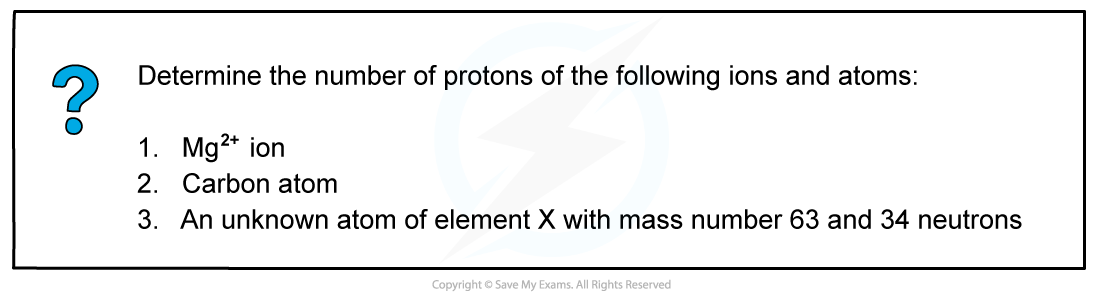
Worked example: Determine the number of protons
Answer
- Answer 1: The atomic number of a magnesium atom is 12 suggesting that the number of protons in the magnesium element is 12
- Therefore the number of protons in a Mg2+ ion is also 12
- Answer 2: The atomic number of a carbon atom is 6 suggesting that a carbon atom has 6 protons in its nucleus
- Answer 3: Use the formula to calculate the number of protons
Number of protons = mass number - number of neutrons
Number of protons = 63 - 34
Number of protons = 29
-
- Element X is therefore copper
Electrons
- An atom is neutral and therefore has the same number of protons and electrons
- Ions have a different number of electrons to their atomic number depending on their charge
- A positively charged ion has lost electrons and therefore has fewer electrons than protons
- A negatively charged ion has gained electrons and therefore has more electrons than protons
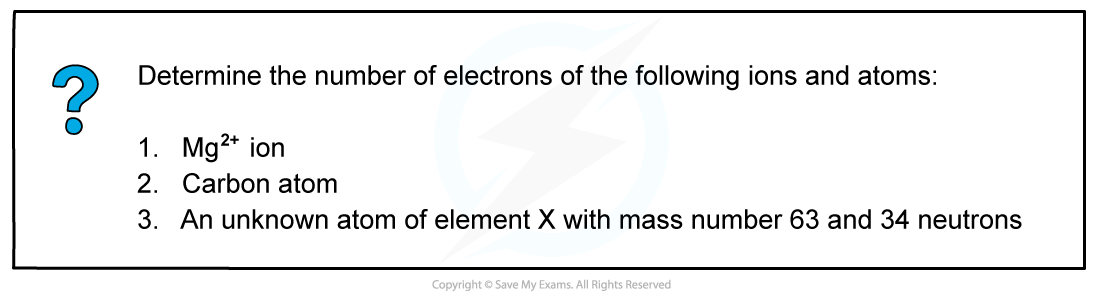
Worked example: Determine the number of electrons
Answer
- Answer 1: The atomic number of a magnesium atom is 12 suggesting that the number of protons in the neutral magnesium atom is 12
- However, the 2+ charge in Mg2+ ion suggests it has lost two electrons
- It only has 10 electrons left now
- Answer 2: The atomic number of a carbon atom is 6 suggesting that the neutral carbon atom has 6 electrons orbiting around the nucleus
- Answer 3: The number of protons of element X can be calculated by:
Number of protons = mass number - number of neutrons
Number of protons = 63 - 34
Number of protons = 29
-
- The neutral atom of element X therefore also has 29 electrons
Neutrons
- The mass and atomic numbers can be used to find the number of neutrons in ions and atoms:
Number of neutrons = mass number (A) - number of protons (Z)
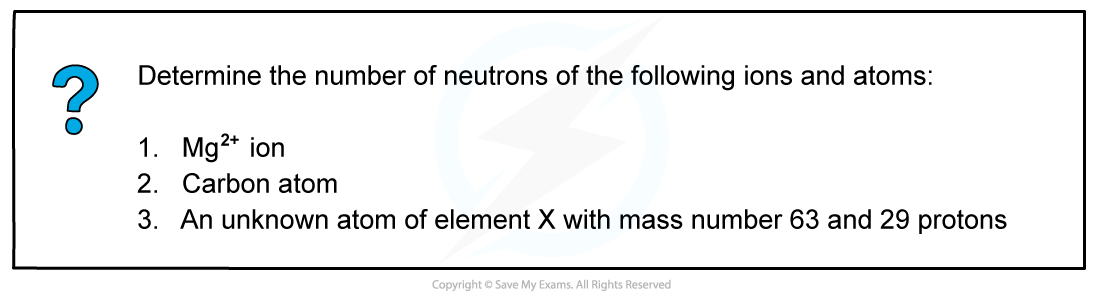
Worked example: Determine the number of neutrons
Answer
- Answer 1: The atomic number of a magnesium atom is 12 and its mass number is 24
Number of neutrons = mass number (A) - number of protons (Z)
Number of neutrons = 24 - 12
Number of neutrons = 12
-
- The Mg2+ ion has 12 neutrons in its nucleus
- Answer 2: The atomic number of a carbon atom is 6 and its mass number is 12
Number of neutrons = mass number (A) - number of protons (Z)
Number of neutrons = 12 - 6
Number of neutrons = 6
-
- The carbon atom has 6 neutrons in its nucleus
- Answer 3: The atomic number of an element X atom is 29 and its mass number is 63
Number of neutrons = mass number (A) - number of protons (Z)
Number of neutrons = 63 - 29
Number of neutrons = 34
-
- The neutral atom of element X has 34 neutrons in its nucleus
转载自savemyexams

在线登记
最新发布
翰林课程体验,退费流程快速投诉邮箱: yuxi@linstitute.net 沪ICP备2023009024号-1








