- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记1.1.1 Particles in the Atom & Atomic Structure
Structure of an Atom
- All matter is composed of atoms, which are the smallest parts of an element that can take place in chemical reactions
- Atoms are mostly made up of empty space around a very small, dense nucleus that contains protons and neutrons
- The nucleus has an overall positive charge
- The protons have a positive charge and the neutrons have a neutral charge
- Negatively charged electrons are found in orbitals in the empty space around the nucleus
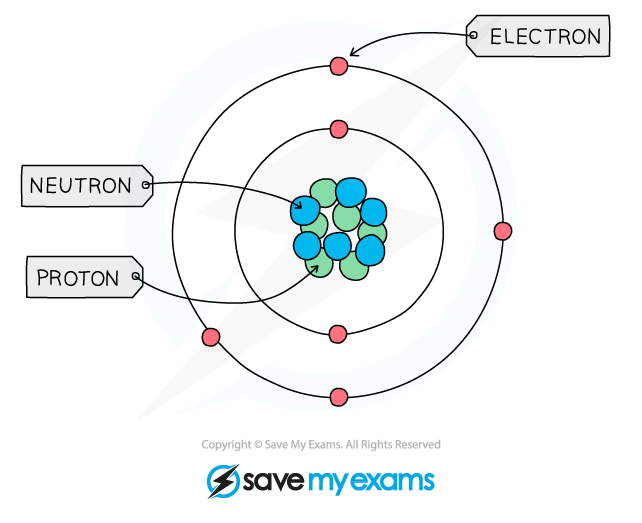
The basic structure of an atom (not to scale)
Subatomic Particles
- The protons, neutrons and electrons that an atom is made up of are called subatomic particles
- These subatomic particles are so small that it is not possible to measure their masses and charges using conventional units (such as grams or coulombs)
- Instead, their masses and charges are compared to each other, and so are called ‘relative atomic masses’ and ‘relative atomic charges’
- These are not actual charges and masses but charges and masses of particles relative to each other
- Protons and neutrons have a very similar mass, so each is assigned a relative mass of 1
- Electrons are 1836 times smaller than a proton and neutron, and so their mass is often described as being negligible
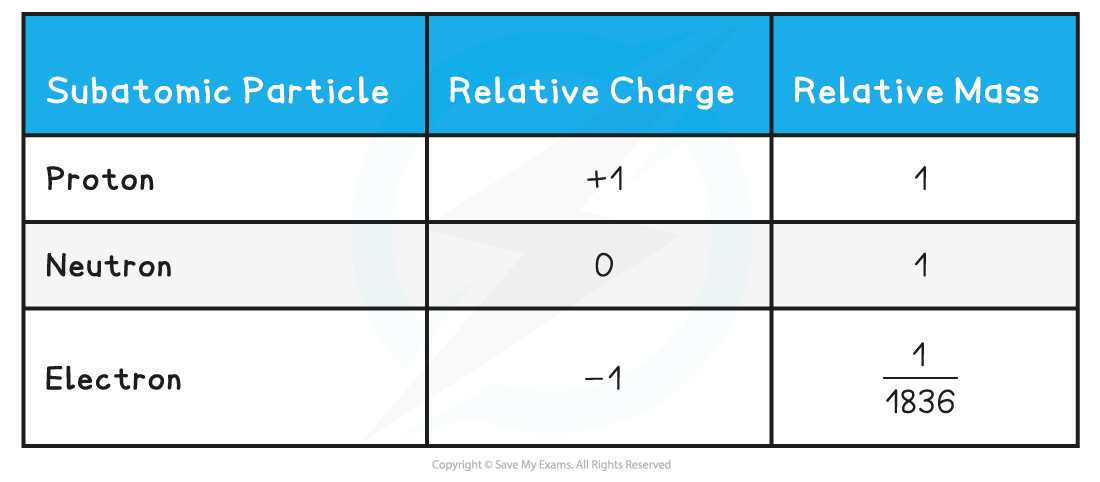
- The relative mass and charge of the subatomic particles are:
Relative mass & charge of subatomic particles table
Exam Tip
You can see from the table how the relative mass of an electron is almost negligibleThe charge of a single electron is -1.602 x 10-19 coulombs, whereas the charge of a proton is +1.602 x 10-19 coulombs. So, relative to each other, their charges are -1 and +1 respectively
Atoms: Key Terms
- The atomic number (or proton number) is the number of protons in the nucleus of an atom and has the symbol Z
- The atomic number is also equal to the number of electrons present in a neutral atom of an element
- E.g. the atomic number of lithium is 3, meaning that a neutral lithium atom has 3 protons and therefore, also has 3 electrons
- The mass number (or nucleon number) is the total number of protons + neutrons in the nucleus of an atom, and has the symbol A
- The number of neutrons can be calculated by:
Number of neutrons = mass number - atomic number
-
- Protons and neutrons are also called nucleons, because they are found in the nucleus
Exam Tip
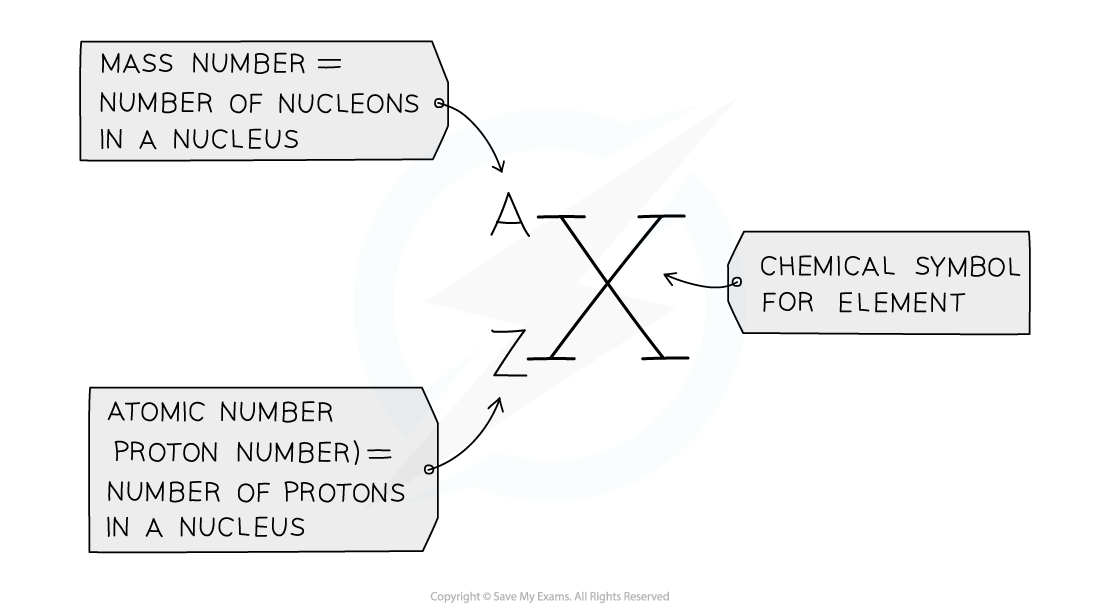
The mass (nucleon) and atomic (proton) number are given for each element in the Periodic Table
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








