- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Biology复习笔记3.2.7 Enzyme Inhibitors
Enzyme Inhibitors
- An enzyme's activity can be reduced or stopped, temporarily, by a reversible inhibitor
- There are two types of reversible inhibitors:
- Competitive inhibitors have a similar shape to that of the substrate molecules and therefore compete with the substrate for the active site
- Non-competitive inhibitors bind to the enzyme at an alternative site, which alters the shape of the active site and therefore prevents the substrate from binding to it

Competitive and non-competitive inhibition
- Reversible inhibitors can act as regulators in metabolic pathways
- Metabolic reactions must be very tightly controlled and balanced, so that no single enzyme can ‘run wild’ and continuously and uncontrollably generate more and more of a particular product
- Metabolic reactions can be controlled by using the end-product of a particular sequence of metabolic reactions as a non-competitive, reversible inhibitor:
- As the enzyme converts substrate to product, the process is itself slowed down as the end-product of the reaction chain binds to an alternative site on the original enzyme, changing the shape of the active site and preventing the formation of further enzyme-substrate complexes
- The end-product can then detach from the enzyme and be used elsewhere, allowing the active site to reform and the enzyme to return to an active state
- This means that as product levels fall, the enzyme begins catalysing the reaction once again, in a continuous feedback loop
- This process is known as end-product inhibition
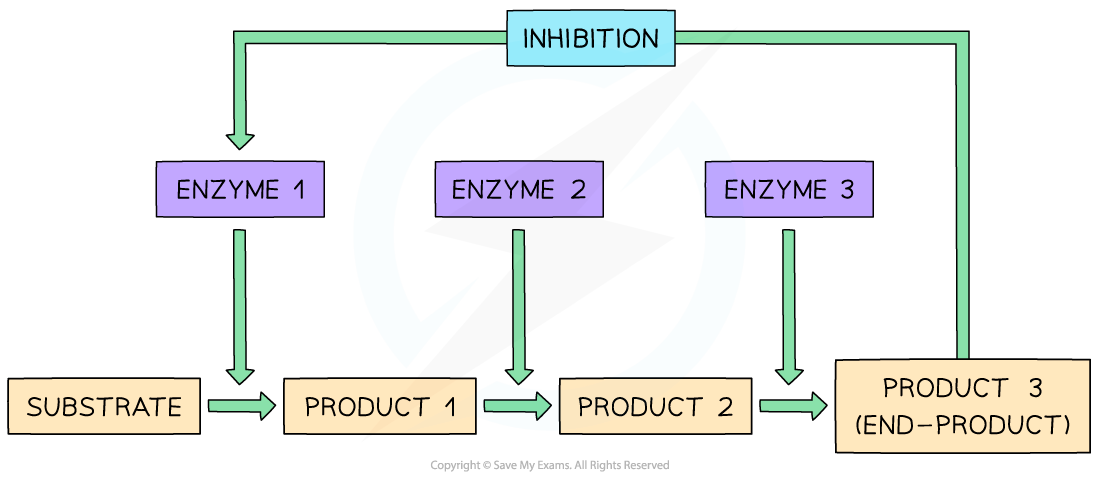
End-product inhibition
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








