- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Biology复习笔记3.1.3 How Enzymes Work
How Enzymes Work
The lock-and-key hypothesis
- Enzymes are globular proteins
- This means their shape (as well as the shape of the active site of an enzyme) is determined by the complex tertiary structure of the protein that makes up the enzyme and is therefore highly specific
- In the 1890’s the first model of enzyme activity was described by Emil Fischer:
- He suggested that both enzymes and substrates were rigid structures that locked into each other very precisely, much like a key going into a lock
- This is known as the ‘lock-and-key hypothesis’
- This was later modified and adapted to our current understanding of enzyme activity, permitted by advances in techniques in the molecular sciences

The lock-and-key hypothesis
The induced-fit hypothesis
- The modified model of enzyme activity is known as the ‘induced-fit hypothesis’
- Although it is very similar to the lock and key hypothesis, in this model the enzyme and substrate interact with each other:
- The enzyme and its active site (and sometimes the substrate) can change shape slightly as the substrate molecule enters the enzyme
- These changes in shape are known as conformational changes
- This ensures an ideal binding arrangement between the enzyme and substrate is achieved
- This maximises the ability of the enzyme to catalyse the reaction
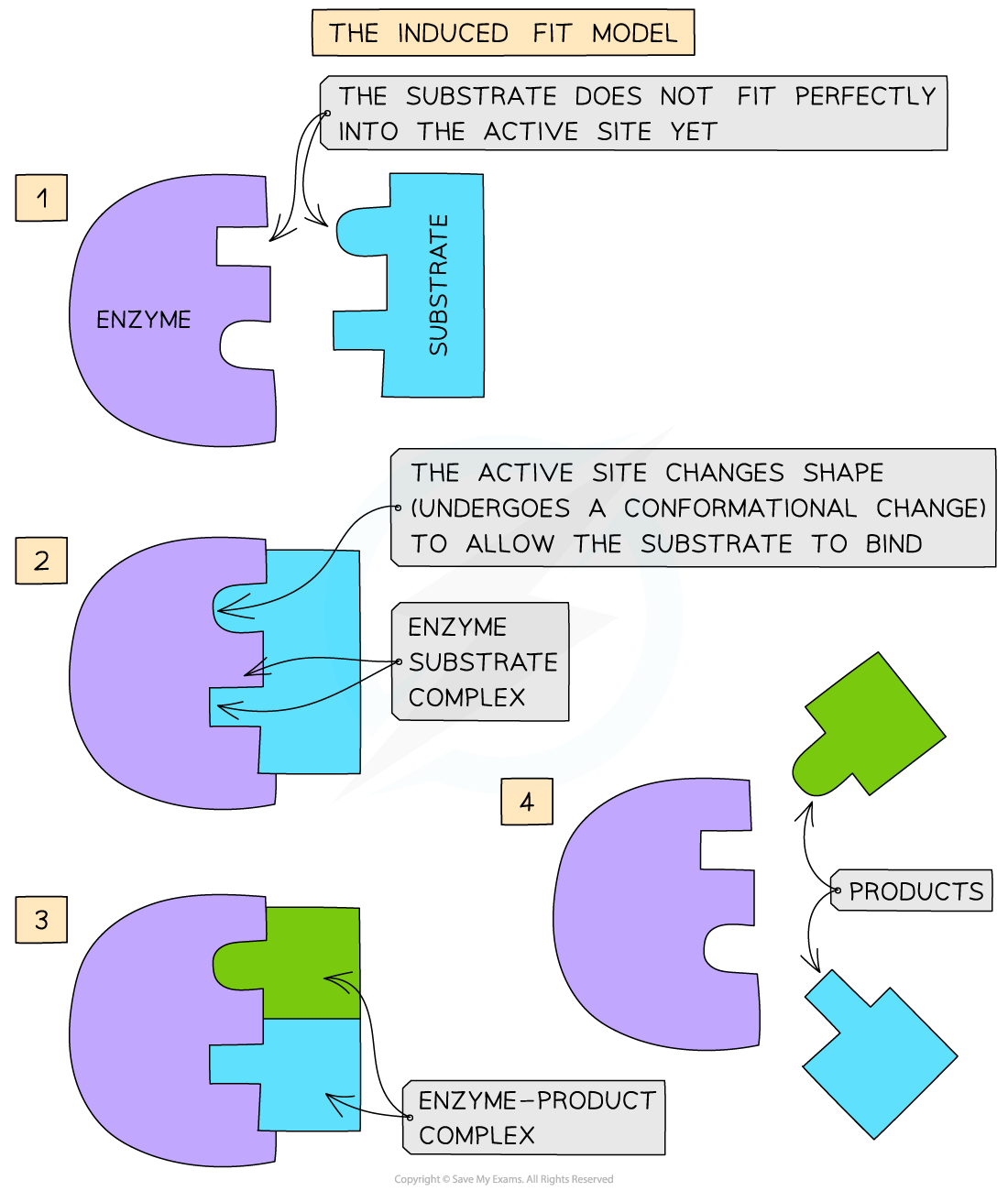
The induced-fit hypothesis
Exam Tip
Don't forget – our current understanding of enzyme-substrate interactions is based on the induced-fit hypothesis.
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








