- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Biology复习笔记2.2.6 Cellulose
Cellulose: Structure & Function
- Cellulose is a polysaccharide
- Polysaccharides are macromolecules that are polymers formed by many monosaccharides joined by glycosidic bonds in a condensation reaction to form chains. These chains may be:
- Branched or unbranched
- Folded (making the molecule compact which is ideal for storage, eg. starch and glycogen)
- Straight (making the molecules suitable to construct cellular structures, eg. cellulose) or coiled
- Polysaccharides are insoluble in water
Cellulose – structure
- Is a polymer consisting of long chains of β-glucose joined together by 1,4 glycosidic bonds
- As β-glucose is an isomer of α-glucose to form the 1,4 glycosidic bonds consecutive β-glucose molecules must be rotated 180° to each other
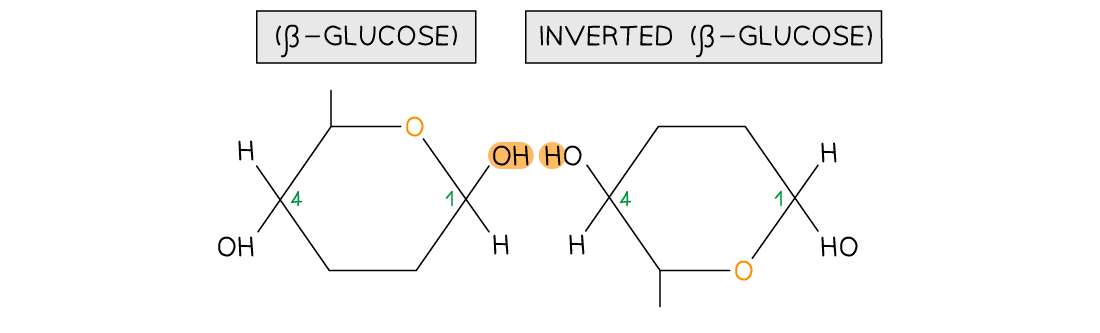
To form the 1,4 glycosidic bond between two β-glucose molecules, the glucose molecules must be rotated to 180° to each other
- Due to the inversion of the β-glucose molecules many hydrogen bonds form between the long chains giving cellulose it’s strength
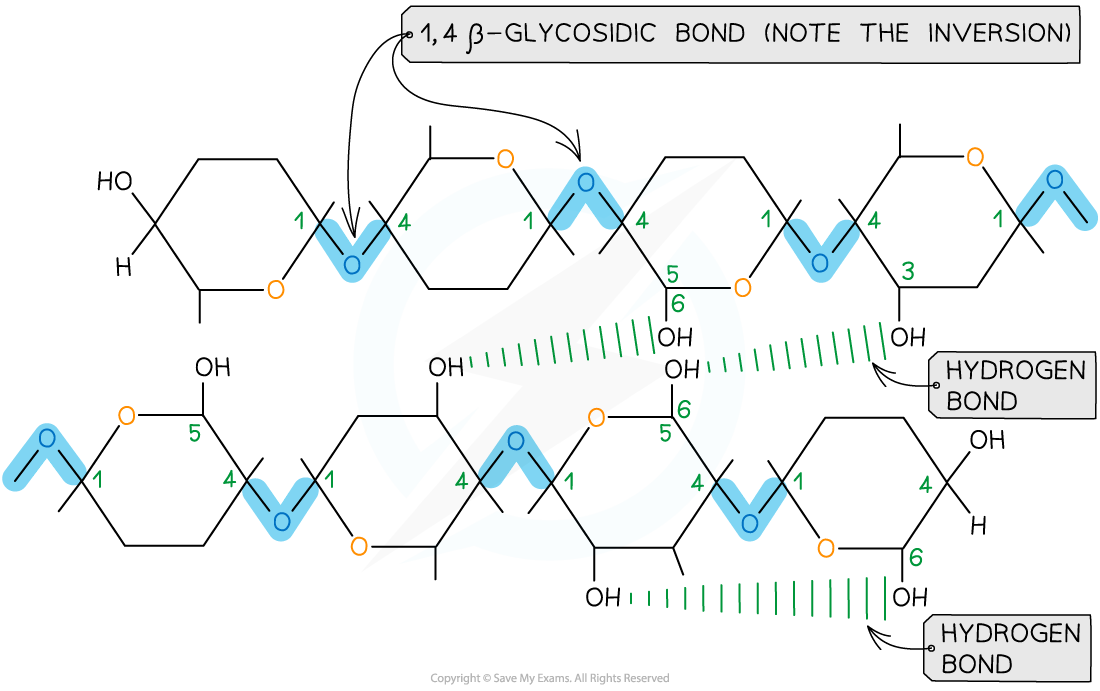
Cellulose is used as a structural component due to the strength it has from the many hydrogen bonds that form between the long chains of β-glucose molecules
Cellulose – function
- Cellulose is the main structural component of cell walls due to its strength which is a result of the many hydrogen bonds found between the parallel chains of microfibrils
- The high tensile strength of cellulose allows it to be stretched without breaking which makes it possible for cell walls to withstand turgor pressure
- The cellulose fibres and other molecules (eg. lignin) found in the cell wall form a matrix which increases the strength of the cell walls
- The strengthened cell walls provides support to the plant
- Cellulose fibres are freely permeable which allows water and solutes to leave or reach the cell surface membrane
- As few organisms have the enzyme (cellulase) to hydrolyse cellulose it is a source of fibre
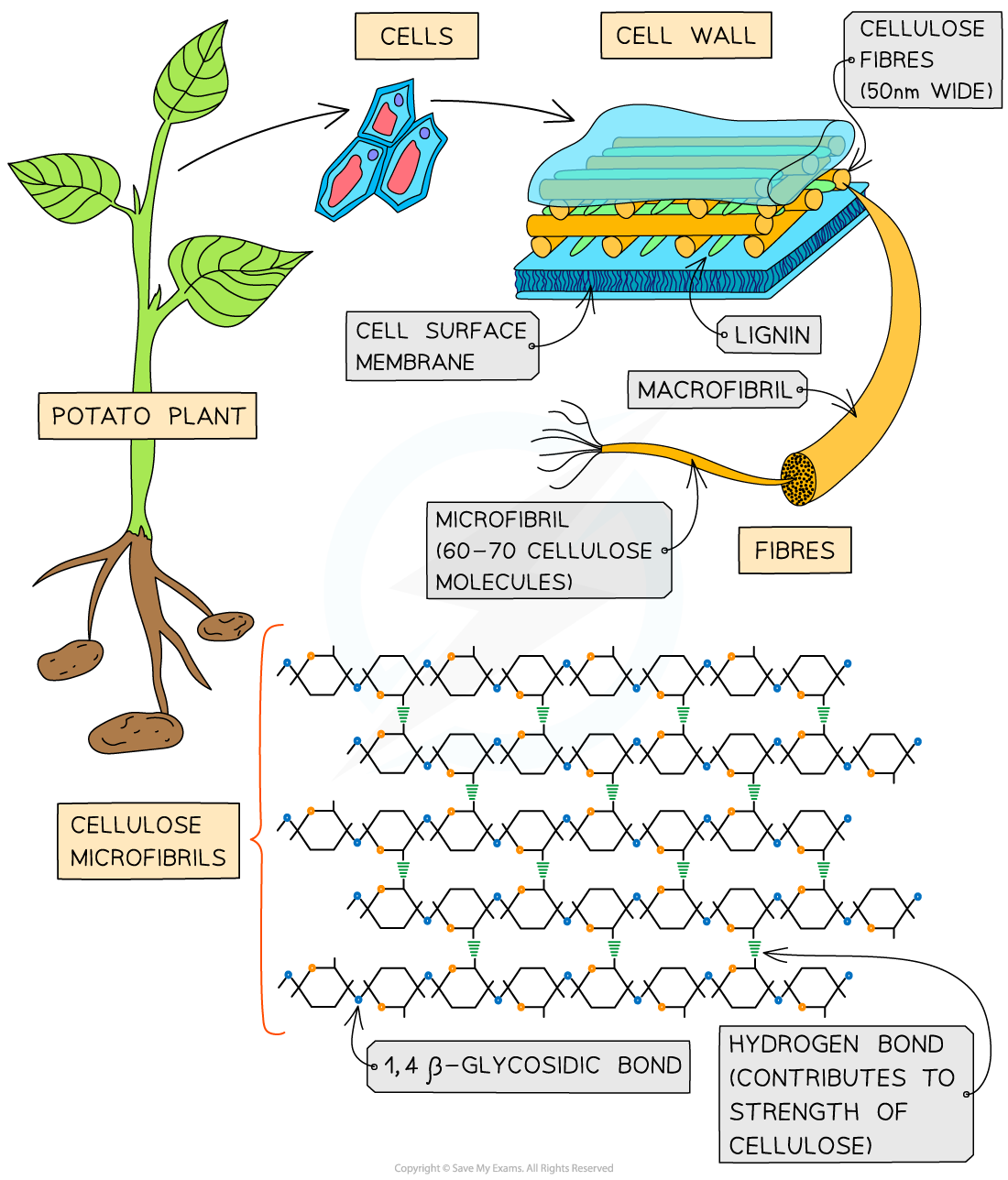
The strength and insolubility of cellulose fibres means it is a suitable molecule to construct cell walls
Exam Tip
Learn the monomer for cellulose, the arrangement of the glycosidic bond (which is dependent on the position of the OH group on carbon 1 and 4) and that cellulose exists in parallel chains bonded by many hydrogen bonds giving it high mechanical strength.
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








