- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Biology复习笔记2.1.3 Testing for Non-Reducing Sugars
Testing for Non-Reducing Sugars
- Sugars can be classified as reducing or non-reducing; this classification is dependent on their ability to donate electrons (a reducing sugar that is able to donate electrons is itself oxidised)
- OILRIG in Chemistry
- If Benedict's test has been carried out on a solution and it shows that no reducing sugars are present then a modified version of the test can be carried out to test for the presence of non-reducing sugars
To test for non-reducing sugars:
- Add dilute hydrochloric acid to the sample and heat in a water bath that has been brought to the boil
- Neutralise the solution with sodium hydrogencarbonate
- Use a suitable indicator (such as red litmus paper) to identify when the solution has been neutralised, and then add a little more sodium hydrogencarbonate as the conditions need to be slightly alkaline for the Benedict’s test to work
- Then carry out Benedict’s test as normal; add Benedict’s reagent to the sample and heat in a water bath that has been boiled – if a colour change occurs (orange-red precipitate), a non-reducing sugar is present
Explanation
- The addition of acid will hydrolyse any glycosidic bonds present in any carbohydrate molecules
- The resulting monosaccharides left will have an aldehyde or ketone functional group that can donate electrons to copper (II) sulfate (reducing the copper), allowing a precipitate to form
Reducing & Non-reducing Sugars Table
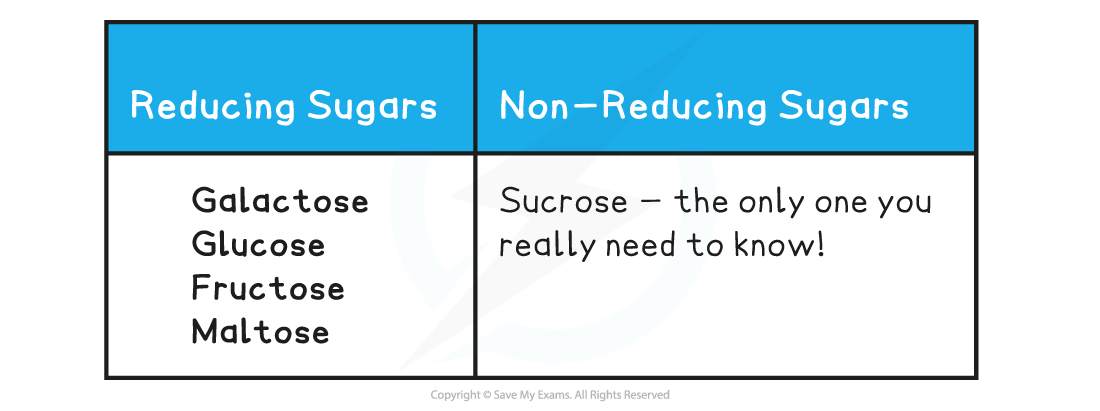
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








