- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Biology复习笔记2.1.2 The Benedict's Test
The Benedict's Test for Reducing Sugars
Method
- Add Benedict's reagent (which is blue as it contains copper (II) sulfate ions) to a sample solution in a test tube
- It is important that an excess of Benedict’s solution is used so that there is more than enough copper (II) sulfate present to react with any sugar present
- Heat the test tube in a water bath or beaker of water that has been brought to a boil for a few minutes
- If a reducing sugar is present, a coloured precipitate will form as copper (II) sulfate is reduced to copper (I) oxide which is insoluble in water
- A positive test result is, therefore, a colour change somewhere along a colour scale from blue (no reducing sugar) to brown/brick-red (a high concentration of reducing sugar)
- This test is semi-quantitative as the degree of the colour change can give an indication of how much (the concentration of) reducing sugar present
Semi-quantitative Benedict's test
- A semi-quantitative test can be carried out by setting up standard solutions with known concentrations of reducing sugar (such as glucose)
- These solutions should be set up using a serial dilution of an existing stock solution
- Each solution is then treated in the same way: add the same volume of Benedict’s solution to each sample and heat in a water bath that has been boiled (ideally at the same temperature each time) for a set time (5 minutes or so) to allow colour changes to occur
- It is important to ensure that an excess of Benedict’s solution is used
- Any colour change observed for each solution of a known concentration in that time can be attributed to the concentration of reducing sugar present in that solution
- The same procedure is carried out on a sample with an unknown concentration of reducing sugar which is then compared to the stock solution colours to estimate the concentration of reducing sugar present
Alterations
- It is also possible to standardise this test but instead of waiting a fixed amount of time for a range of colours to be observed, time how long it takes for the first colour change to occur (blue to green)
- The higher the concentration of reducing sugar in a sample, the less time it would take for a colour change to be observed
- To avoid issues with human interpretation of colour, a colourimeter could be used to measure the absorbance or transmission of light through the sugar solutions of known concentration to establish a range of values that an unknown sample can be compared against a calibration curve
Serial dilutions
- Serial dilutions are created by taking a series of dilutions of a stock solution. The concentration decreases by the same quantity between each test tube
- They can either be ‘doubling dilutions’ (where the concentration is halved between each test tube) or a desired range (e.g. 0, 2, 4, 6, 8, 10 mmol dm-3)
- Serial dilutions are completed to create a standard to compare unknown concentrations against
- The comparison can be:
- Visual
- Measured through a calibration/standard curve
- Measured using a colourimeter
- They can be used when:
- Counting bacteria or yeast populations
- Determining unknown glucose, starch, protein concentrations
- The comparison can be:
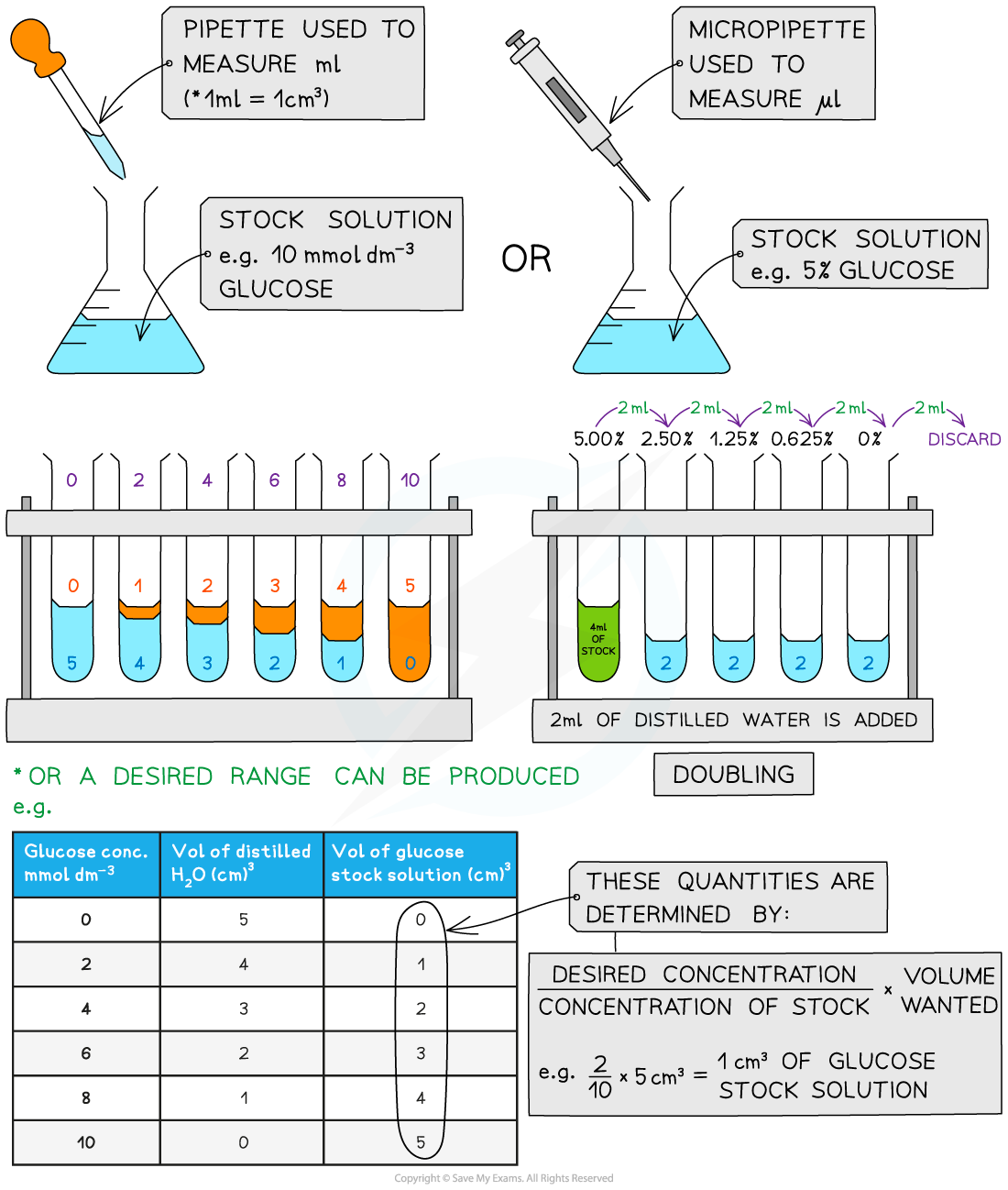
Making serial dilutions
Colourimeter
- A colourimeter is an instrument that beams a specific wavelength (colour) of light through a sample and measures how much of this light is absorbed (arbitrary units)
- They provide a quantitative measurement
- They contain different wavelengths or colour filters (depends on the model of colourimeter), so that a suitable colour can be shone through the sample and will not get absorbed. This colour will be the contrasting colour (eg. a red sample should have green light shone through)
- Remember that a sample will look red as that wavelength of light is being reflected but the other wavelengths will be absorbed
- Colourimeters must be calibrated before taking measurements
- This is completed by placing a blank into the colourimeter and taking a reference, it should read 0 (that is, no light is being absorbed)
- This step should be repeated periodically whilst taking measurements to ensure that the absorbance is still 0
- The results can then be used to plot a calibration or standard curve
- Absorbance against the known concentrations can be used
- Unknown concentrations can then be determined from this graph
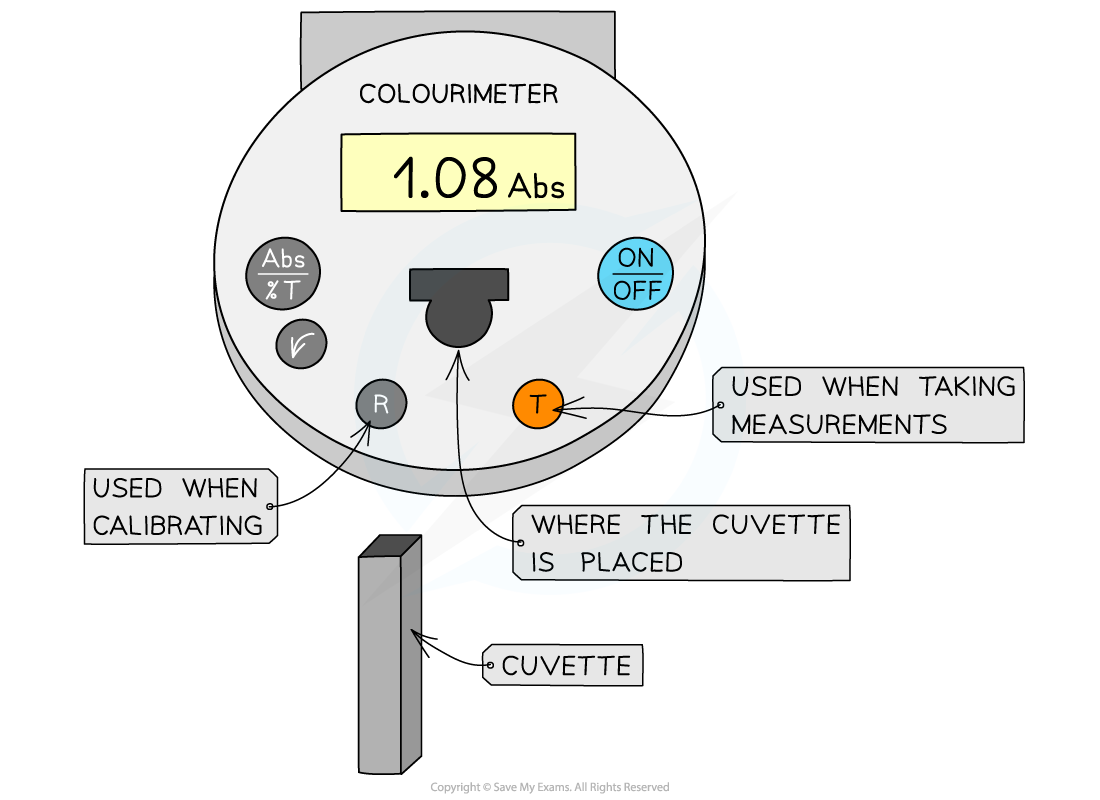
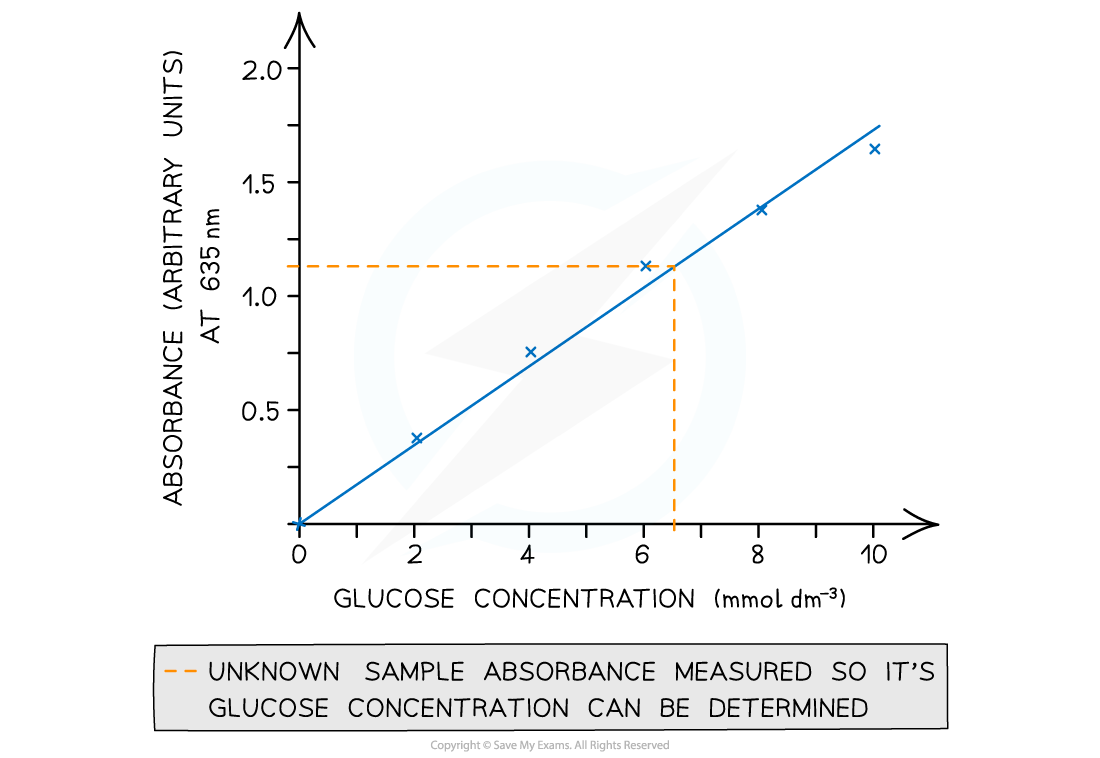
A colourimeter is used to obtain quantitative data that can be plotted to create a calibration curve to be used to find unknown concentrations
转载自savemyexams


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








