- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Physics: SL复习笔记3.1.5 Specific Latent Heat
Specific Latent Heat
- During a phase change (i.e. a change of state) thermal energy is transferred to a substance or removed from it, while the temperature of the substance does not change
- In this case, the thermal energy is calculated as follows:
Q = mL
- Where:
- Q = heat energy transferred (J)
- m = mass of the substance in kilograms (kg)
- L = specific latent heat of the substance in J kg–1
- The specific latent heat of a substance is defined as:
The amount of energy required to change the state of 1 kg of a substance without changing its temperature
- This definition can be explained when the above equation is rearranged for L:
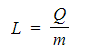 This means that the higher the specific latent heat of a substance, the greater the energy needed to change its state
This means that the higher the specific latent heat of a substance, the greater the energy needed to change its state
- Note that the specific latent heat is measured in J kg–1
- The amount of energy required to melt (or solidify) a substance is not the same as the amount of energy required to evaporate (or condense) the same substance
- Hence, there are two types of specific heat:
- Specific latent heat of fusion, Lf
- Specific latent heat of vaporisation, Lv
- Specific latent heat of fusion is defined as:
The energy released when 1 kg of liquid freezes to become solid at constant temperature
- This applies to the following phase changes:
- Solid to liquid
- Liquid to solid
- Therefore, the definition for specific latent heat of fusion could also be:
The energy absorbed when 1 kg of solid melts to become liquid at constant temperature
- Specific latent heat of vaporisation is defined as:
The energy released when 1 kg of gas condenses to become liquid at constant temperature
- This applies to the following phase changes:
- Liquid to gas
- Gas to liquid
- Therefore, the definition for specific latent heat of vaporisation could also be:
The energy absorbed when 1 kg of liquid evaporates to become gas at constant temperature
- For the same substance, the value of the specific latent heat of vaporisation is always much higher than the value of the specific latent heat of fusion
- In other words, Lv > Lf
- This is because much more energy is needed to evaporate (or condense) a substance than it is needed to melt it (or solidify it)
- In melting, the intermolecular bonds only need to be weakened to turn from a solid to a liquid
- When evaporating, the intermolecular bonds need to be completely broken to turn from liquid to gas. This requires a lot more energy.
Worked Example
Determine the energy needed to melt 200 g of ice at 0°C.
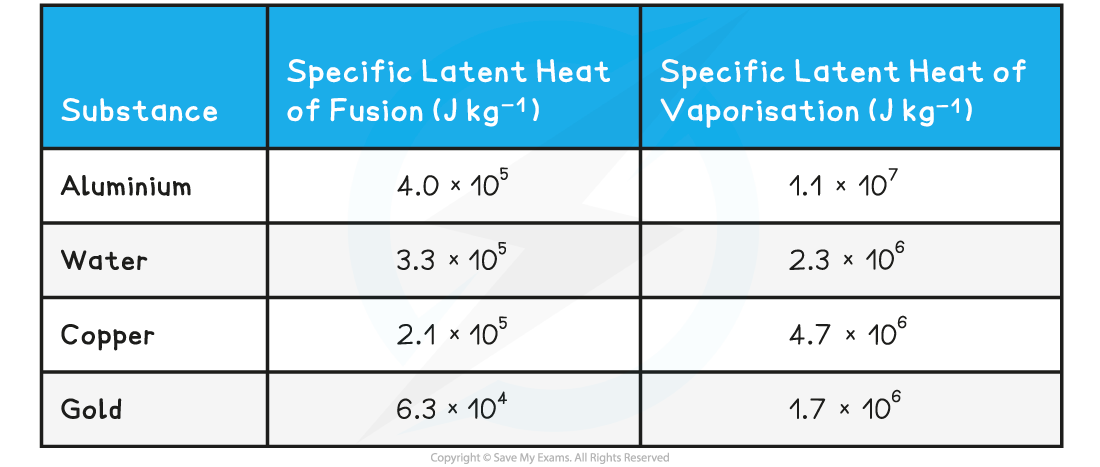
- The specific latent heat of fusion of water is 3.3 × 105 J kg–1
- The specific latent heat of vaporisation of water is 2.3 × 106 J kg–1
Step 1: Determine whether to use latent heat of fusion or vaporisation
-
- We need to use the specific latent heat of fusion because the phase change occurring is from solid to liquid
Step 2: List the known quantities
-
- Mass of the ice, m = 200 g = 0.2 kg
- Specific latent heat of fusion of water, Lf = 3.3 × 105 J kg–1
Step 3: Write down the equation for the thermal energy
Q = mLf
Step 4: Substitute numbers into the equation
Q = 0.2 kg × (3.3 × 105) J kg–1
Q = 6.6 × 104 J = 66 kJ
Worked Example
Energy is supplied to a heater at a rate of 2500 W.Determine the time taken to boil 0.50 kg of water at 100°C. Ignore energy losses.
- The specific latent heat of fusion of water is 3.3 × 105 J kg–1
- The specific latent heat of vaporisation of water is 2.3 × 106 J kg–1
Step 1: Determine whether to use latent heat of fusion or vaporisation
-
- We need to use the specific latent heat of vaporisation because the phase change occurring is from liquid to gas
Step 2: Write down the known quantities
-
- Power, P = 2500 W
- Mass, m = 0.50 kg
- Specific latent heat of vaporisation of water, Lv = 2.3 × 106 J kg–1
Step 3: Recall the equation linking power P, energy E and time t
E = Pt
Step 4: Write down the equation for the thermal energy E
-
- The energy E in the previous equation is the thermal energy Q transferred by the heater to the water
Q= mLf
Step 5: Equate the two expressions for energy
Pt = mLf
Step 6: Solve for the time t
![]()
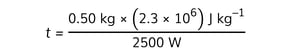
t = 460 s
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









