- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Physics: SL复习笔记3.1.1 Solids, Liquids & Gases
Solids, Liquids & Gases
- The three states of matter are solid, liquid and gas
- The kinetic theory of matter is a model that attempts to explain the properties of the three states of matter
- In this model, particles are assumed to be small solid spheres
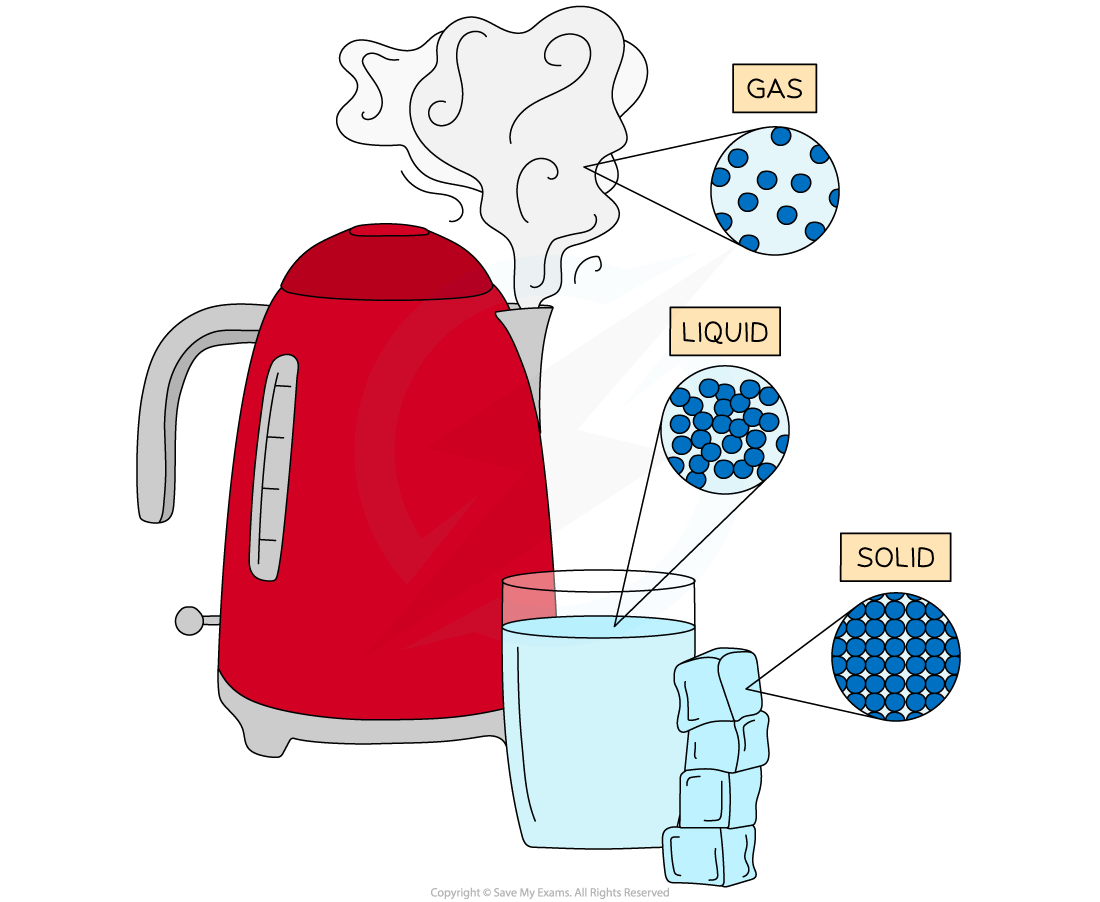
Water has three states of matter; solid ice, liquid water and gaseous steam. The difference between each state is the arrangement of the particles
Solids
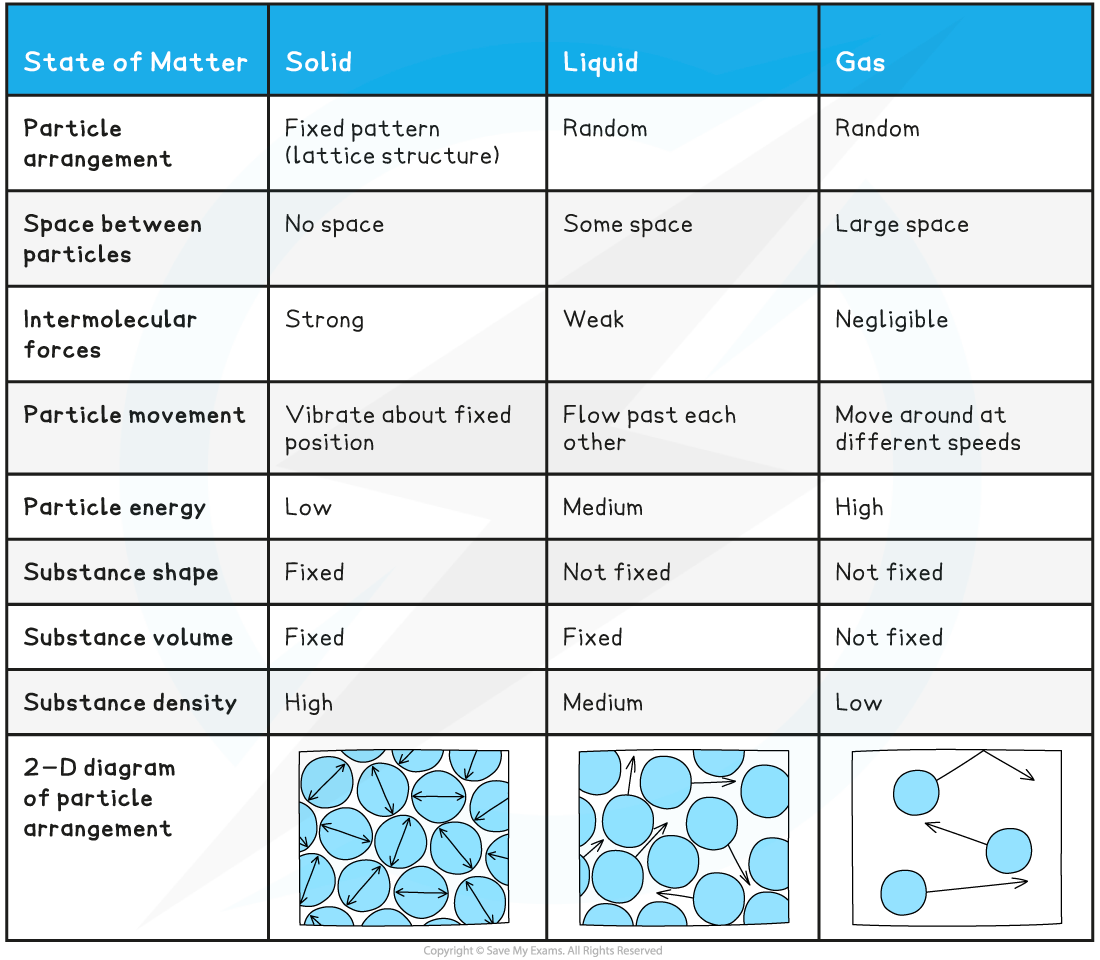
- Particles in solids:
- Are held together by strong intermolecular forces
- Are closely packed
- Are arranged in a fixed pattern (lattice structure)
- Can only vibrate about their fixed positions
- Have low energies compared to particles in liquids and gases
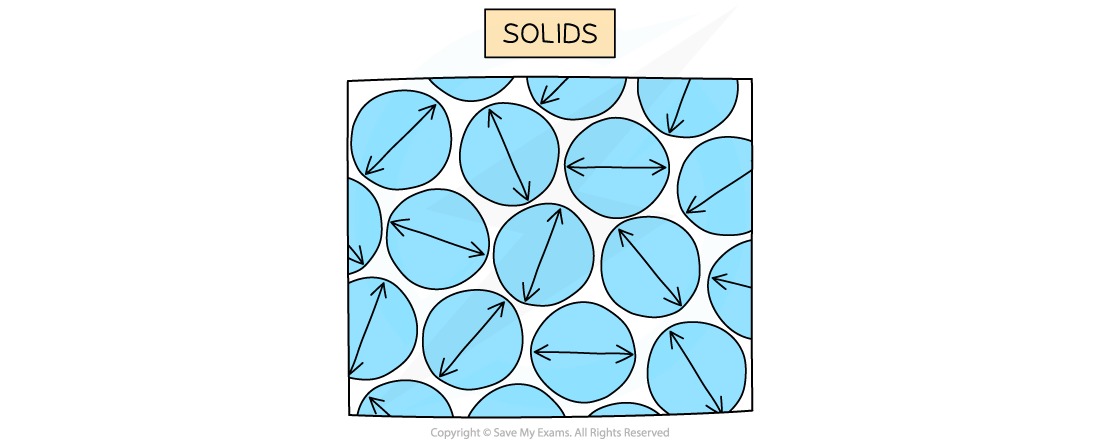
In a solid, particles are arranged in a fixed pattern, with no spaces between them, and are only able to vibrate about their fixed positions
- As a result of the arrangement and behaviour of their particles, solids:
- Have a fixed shape (although some solids can be deformed when forces are applied)
- Have a fixed volume
- Are very difficult to compress
- Have higher densities than liquids and gases
Liquids
- Particles in liquids:
- Are held together by weaker intermolecular forces compared to the forces between particles in solids
- Are closely packed
- Are randomly arranged (i.e. there is no fixed pattern)
- Can flow past each other
- Have higher energies than particles in solids, but lower energies than gas particles
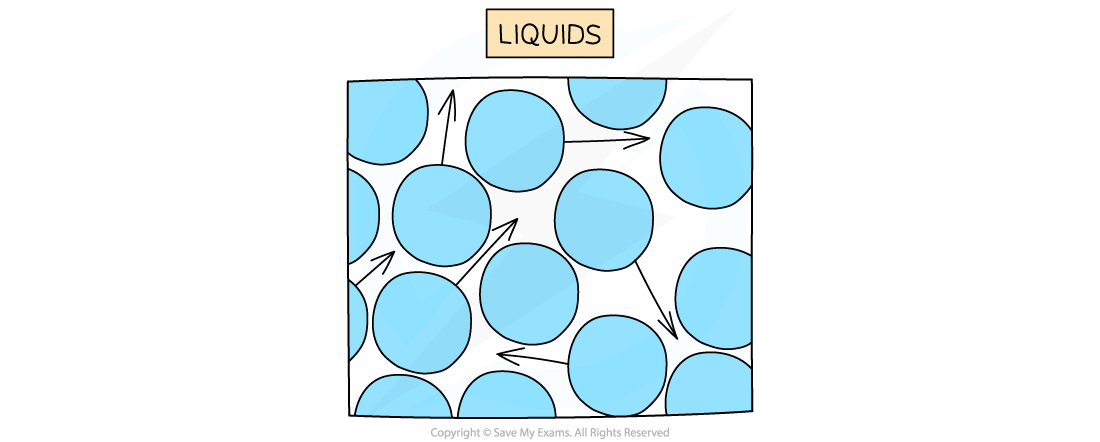
In a liquid, particles are arranged randomly and are able to flow past one another
- As a result of the arrangement and behaviour of their particles, liquids:
- Do not have a fixed shape and take the shape of the container they are held in
- Have a fixed volume
- Are difficult to compress
- Have lower densities than solids, but higher densities than gases
Gases
- Particles in gases:
- Have negligible intermolecular forces between them
- Are far apart (the average distance between the particles is ∼10 times greater than the distance between the particles in solids and liquids)
- Are randomly arranged
- Move around in all directions at a variety of speeds, occasionally colliding with each other and with the walls of the container they are in
- Are negligible in size compared to the volume occupied by the gas
- Have higher energies than particles in solids and liquids
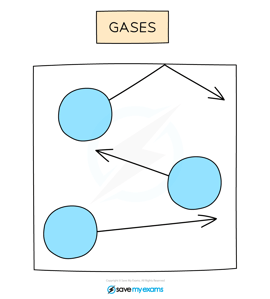
In a gas, particles can move around freely in all directions (shown by the arrows).
- As a result of the arrangement and behaviour of their particles, gases:
- Do not have a fixed shape and take the shape of the container they are held in
- Do not have a fixed volume and expand to completely fill the available volume
- Can be compressed
- Have the lowest densities (∼1000 times smaller than the densities of solids and liquids)
Worked Example
Liquids are about 1000 times denser than gases. Let d be the diameter of a molecule. Estimate the average intermolecular distance in a gas. Give your answer in terms of d.
Step 1: Recall the equation for density
![]()
Step 2: Write down the relationship between the density of a gas ρgas and the density of a liquid ρliquid
ρliquid = 1000 ρgas
Step 3: Write down the relationship between the volume of a liquid Vliquid and the volume of a gas Vgas
-
- Since the mass stays the same, the relationship between the densities translates into a relationship between volumes
Vliquid = 1000 Vgas
Step 4: Relate the volume to the average distance between the molecules, x
-
- The average distance x between the molecules is related to the cube root of the volume
x = ∛1000 Vliquid= 10d
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









