- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: SL复习笔记9.1.9 Voltaic Cells
Voltaic Cells
- Voltaic (or Galvanic) cells generate electricity from spontaneous redox reactions
- For example:
Zn (s) + CuSO4 (aq)→ Cu (s) + ZnSO4 (aq)
- Instead of electrons being transferred directly from the zinc to the copper ions a cell is built which separates the two redox processes
- Each part of the cell is called a half cell
- If a rod of metal is dipped into a solution of its own ions, an equilibrium is set up
- For example:
Zn (s) ⇌ Zn2+ (aq) + 2e-
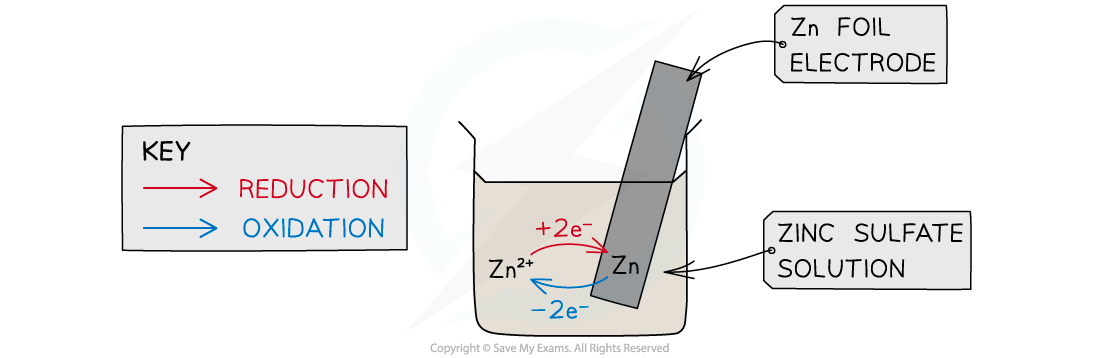
When a metal is dipped into a solution contains its ions an equilibrium is established between the metal and it ions
- This is a half cell and the strip of metal is an electrode
- The position of the equilibrium determines the potential difference between the metal strip and the solution of metal
- The Zn atoms on the rod can deposit two electrons on the rod and move into solution as Zn2+ ions:Zn(s) ⇌ Zn2+(aq) + 2e-
- This process would result in an accumulation of negative charge on the zinc rod
- Alternatively, the Zn2+ ions in solution could accept two electrons from the rod and move onto the rod to become Zn atoms:Zn2+(aq) + 2e- ⇌ Zn(s)
- This process would result in an accumulation of positive charge on the zinc rod
- In both cases, a potential difference is set up between the rod and the solution
- This is known as an electrode potential
- A similar electrode potential is set up if a copper rod is immersed in a solution containing copper ions (eg CuSO4), due to the following processes:
Cu2+(aq) + 2e- ⇌ Cu(s) - reduction (rod becomes positive)
Cu(s) ⇌ Cu2+(aq) + 2e- - oxidation (rod becomes negative)
- Note that a chemical reaction is not taking place - there is simply a potential difference between the rod and the solution
- The potential difference will depend on
- the nature of the ions in solution
- the concentration of the ions in solution
- the type of electrode used
- the temperature
Creating an emf
- If two different electrodes are connected, the potential difference between the two electrodes will cause a current to flow between them. Thus an electromotive force (emf) is established and the system can generate electrical energy
- A typical electrochemical cell can be made by combining a zinc electrode in a solution of zinc sulphate with a copper electrode in a solution of copper sulphate
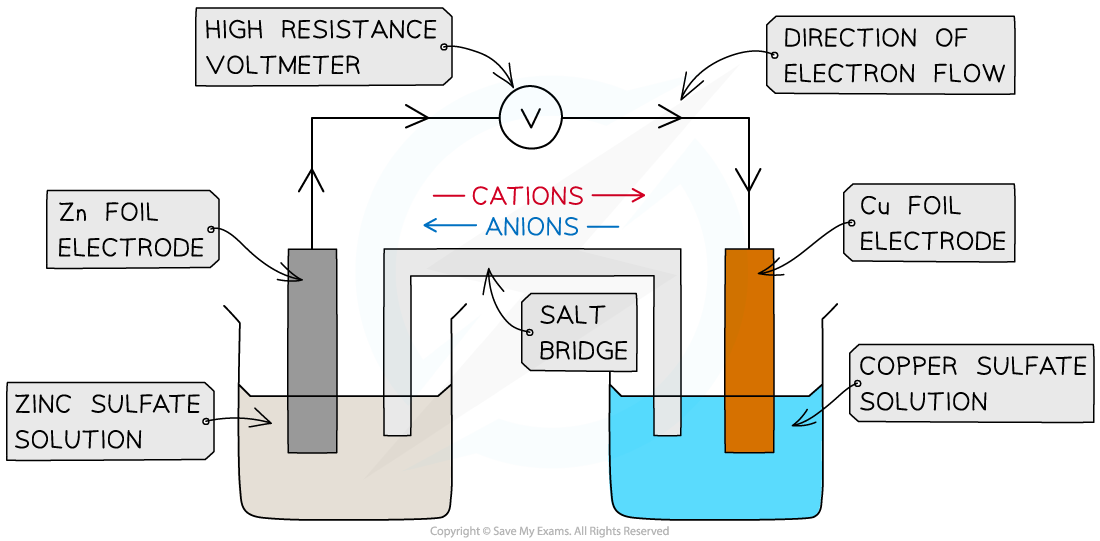
The zinc-copper voltaic cell ( also known as the Daniell Cell)
- The circuit must be completed by allowing ions to flow from one solution to the other
- This is achieved by means of a salt bridge - often a piece of filter paper saturated with a solution of an inert electrolyte such as KNO3(aq)
- The e.m.f can be measured using a voltmeter
- Voltmeters have a high resistance so that they do not divert much current from the main circuit
- The combination of two electrodes in this way is known as an electrochemical or voltaic cell, and can be used to generate electricity
- The positive electrode or cathode is the one which most favours reduction
- In this case it is the copper electrode which is positive
- The negative electrode or anode is the one which most favours oxidation
- In this case it is the zinc electrode which is negative
- Thus electrons flow from the zinc electrode to the copper electrode
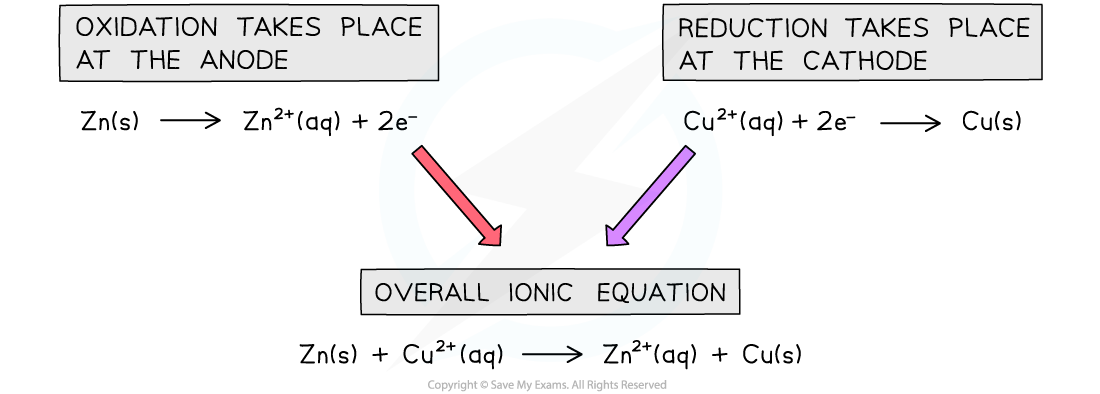
Ionic equations for the Daniell Cell
- To maintain charge in the half cells:
- anions flow to the negative half cell to replace the negative charge of the electrons
- cations flow to the positive half cell since there is a surplus positive charge from the metals becoming cations in the negative half cell
- The sulphate ions flow through the salt bridge from the Cu2+(aq) solution to the Zn2+(aq) solution, to complete the circuit and compensate for the reduced Cu2+ concentration and increased Zn2+ concentration
- The overall cell reaction including spectator ions can thus be written as follows:
Zn (s) + CuSO4 (aq)→ Cu (s) + ZnSO4 (aq)
- The external connection must be made of a metallic wire in order to allow electrons to flow
- The salt bridge must be made of an aqueous electrolyte to allow ions to flow
- By allowing two chemical reagents to be connected electrically, but not chemically, a reaction can only take place if the electrons flow externally
- The chemical energy is thus converted into electrical energy.
Exam Tip
Students often confuse the redox process that take place in voltaic cells and electrolytic cells. An easy way to remember is the phrase RED CATS:REDuction takes place at the CAThode !
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









