- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: SL复习笔记9.1.5 Half Equations
Half Equations
- Oxidation numbers can be used to balance chemical equations
- Go through these steps to balance a redox equation:
-
- Write the unbalanced equation and identify the atoms which change in ox. no.
- Deduce the ox.no. changes
- Balance the ox.no. changes
- Balance the charges
- Balance the atoms
Worked Example
Manganate(VII) ions (MnO4-) react with Fe2+ ions in the presence of acid (H+) to form Mn2+ ions, Fe3+ ions and waterWrite the overall redox equation for the reaction
Answer:
Step 1: Write the unbalanced equation and identify the atoms which change in ox. no.
 Step 2: Deduce the ox.no. changes
Step 2: Deduce the ox.no. changes
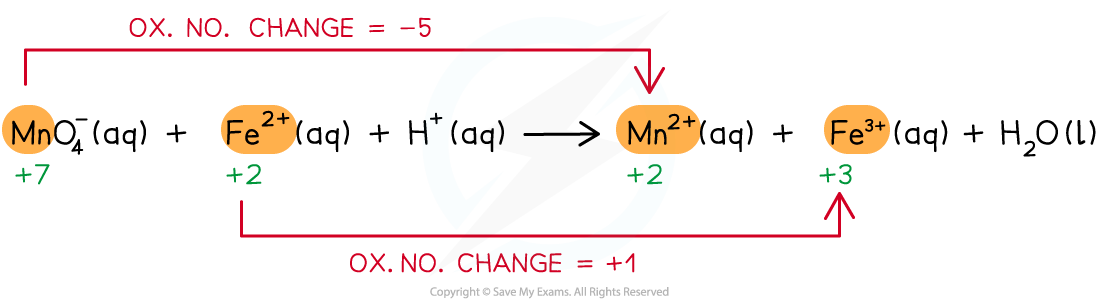
Step 3: Balance the ox.no. changes
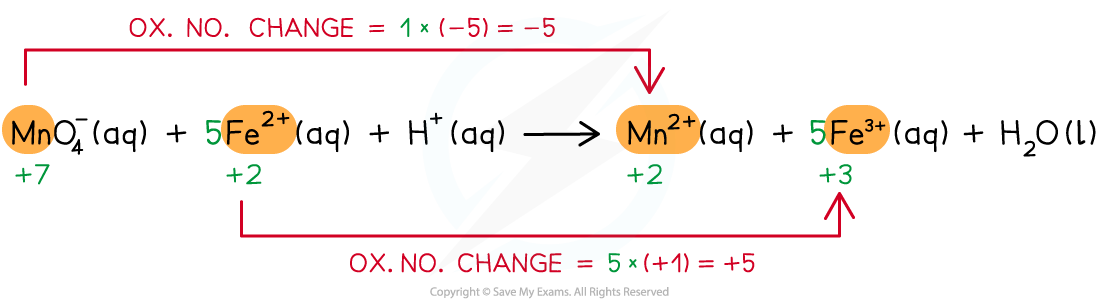
Step 4: Balance the charges
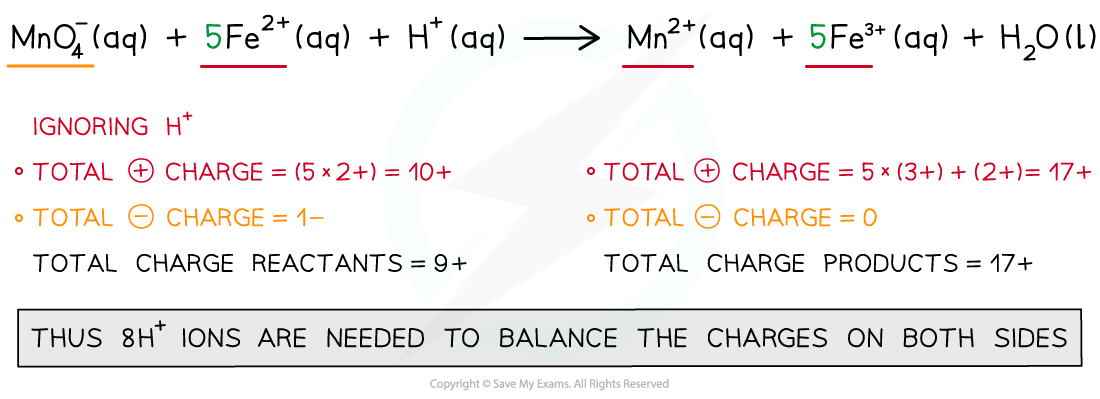
Step 5: Balance the atoms

转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








