- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: SL复习笔记7.1.6 Catalysts & Equilibrium
Catalysts & Equilibrium
Changes in concentration
- If all other conditions stay the same, the equilibrium constant Kc is not affected by any changes in concentration of the reactants or products
- For example, the decomposition of hydrogen iodide:
2HI ⇌ H2 + I2
The equilibrium expression is:
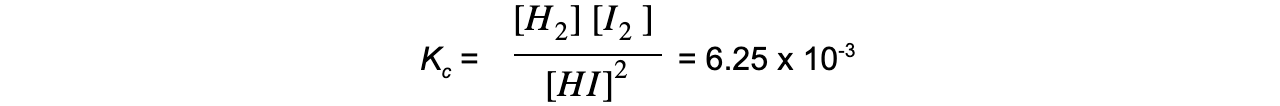
Adding more HI makes the ratio of [ products ] to [ reactants ] smaller
To restore equilibrium, [H2] and [I2] increases and [HI] decreases
Equilibrium is restored when the ratio is 6.25 x 10-3 again
Changes in pressure
- A change in pressure only changes the position of the equilibrium (see Le Chatelier’s principle)
- If all other conditions stay the same, the equilibrium constant Kc is not affected by any changes in pressure of the reactants and products
Changes in temperature
- Changes in temperature change the equilibrium constant Kc
- For an endothermic reaction such as:
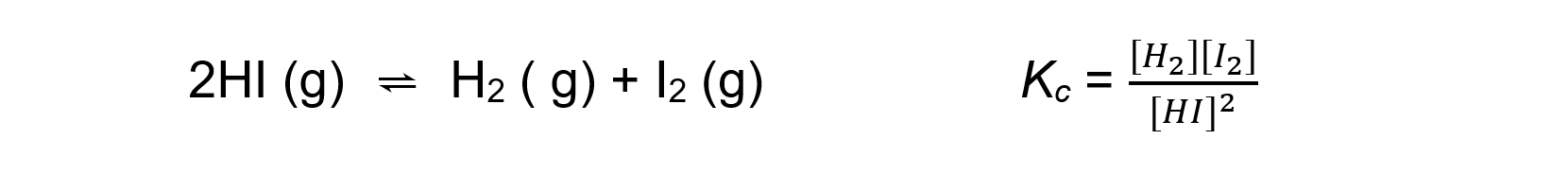
An increase in temperature:
[H2] and [I2] increases
[HI] decreasesBecause [H2] and [I2] are increasing and [HI] is decreasing, the equilibrium constant Kc increases
- For an exothermic reaction such as:
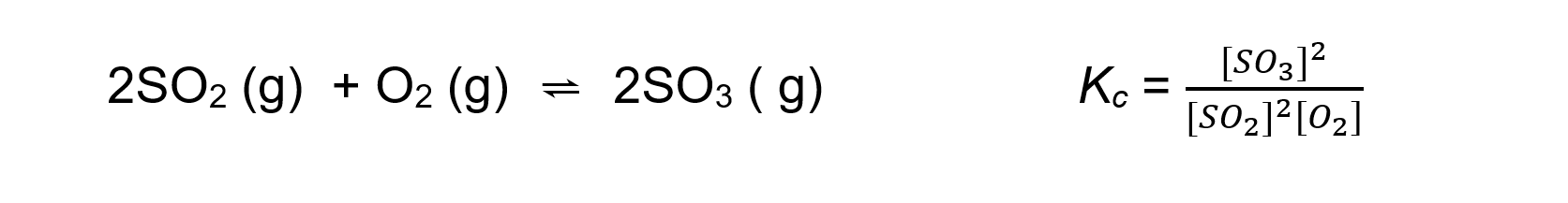
An increase in temperature:
[SO3] decreases
[SO2] and [O2] increasesBecause [SO3] decreases and [SO2] and [O2] increases the equilibrium constant Kc decreases
Presence of a catalyst
- If all other conditions stay the same, the equilibrium constant Kc is not affected by the presence of a catalyst
- A catalyst speeds up both the forward and reverse reactions at the same rate so the ratio of [ products ] to [ reactants ] remains unchanged
Worked Example
An equilbrium is established in the following reaction:
AB (aq) + CD (aq) ⇌ AC (aq) + BD (aq) ∆H = + 180 kJ mol-1
Which factors would affect the value of in this equilibrium?
Answer:
-
- Only a change in temperature will affect the value of Kc and any other changes in conditions would result in the position of the equilibrium moving in such way to oppose this change.
- Adding a catalyst will increase the rate of reaction meaning the state of equilibrium will be reached faster but will have no effect on the position of the equilibrium and therefore Kc is unchanged.
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








