- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: SL复习笔记5.2.4 Using ∆Hf° to Find Enthalpy Changes
Using ∆Hf° to Find Enthalpy Changes
- Standard Enthalpy of Formation is defined as
“The enthalpy change when one mole of a compound is formed from its elements under standard conditions”
- We can use enthalpy of formation of substances to find an unknown enthalpy change using a Hess cycle
- In this type of cycle the elements are always placed at the bottom of the diagram

Enthalpy changes using enthalpy of formation
- In this cycle the arrows will always be pointing upwards because the definition of the enthalpy of formation must go from elements to compounds
- This means the Hess's Law calculation of ΔH will always be in the same arrangement

- Try the following worked example:
Worked Example
Given the data: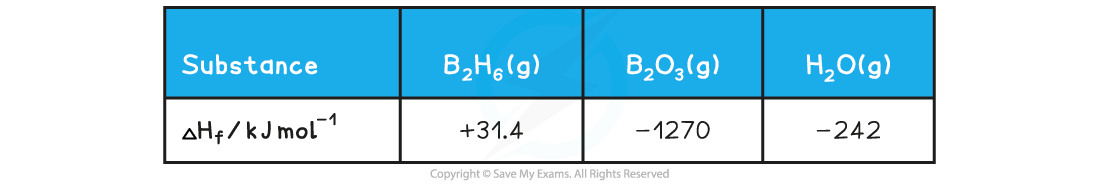 Calculate the enthalpy of combustion of gaseous diborane given that it burns according to the following equation:
Calculate the enthalpy of combustion of gaseous diborane given that it burns according to the following equation:
B2H6(g) + 3O2(g) → B2O3(s) + 3H2O(g)
Answer
Step 1: Find the sum of the enthalpies of combustion of the products
ΔHf = + ( -1270) + ( - 242 x 3) = - 1996 kJ
Step 2: Find the sum of the enthalpies of combustion of the reactants
ΔHf = + (+31.4) + 0 = + 31.4 kJ
There is no enthalpy of formation for oxygen as ΔHf of elements by definition is zero
Step 3: Calculate the enthalpy change
ΔH = ΔHf products - ΔHf reactants = - 1996 - (+ 31.4) = -2027.4 kJ
Exam Tip
In Paper 1, Enthalpy of Formation data will given in the question. For Paper 2, you may need to refer to Section 12 of the Data Booklet where you will find Thermodynamic Data for Selected Compounds
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








