- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
IB DP Chemistry: SL复习笔记4.3.5 Trends in Melting Points of Metals
Trends in Melting Points of Metals
- The strength of electrostatic attraction can be increased by:
- Increasing the number of delocalised electrons per metal atom
- Increasing the positive charges on the metal centres in the lattice
- Decreasing the size of the metal ions
- These factors can be seen in the trends across a period and down a group
Melting points of metals across a period
- If you compare the electron configuration of sodium, magnesium and aluminium you can see the number of valence electrons increases
- Na = 1s22s22p63s1
- Mg = 1s22s22p63s2
- Al = 1s22s22p63s23p1
- Aluminium ions are also a smaller size than magnesium ions or sodium ions and these two factors lead to stronger metallic bonding which can be seen in the melting points
- The stronger the metallic bonding, the more energy is need to break the metallic lattice and so the higher the melting point
- As we go across period 3 we can see the effect of stronger metallic bonding on the metals
- Remember only the first three elements have metallic bonding in this graph
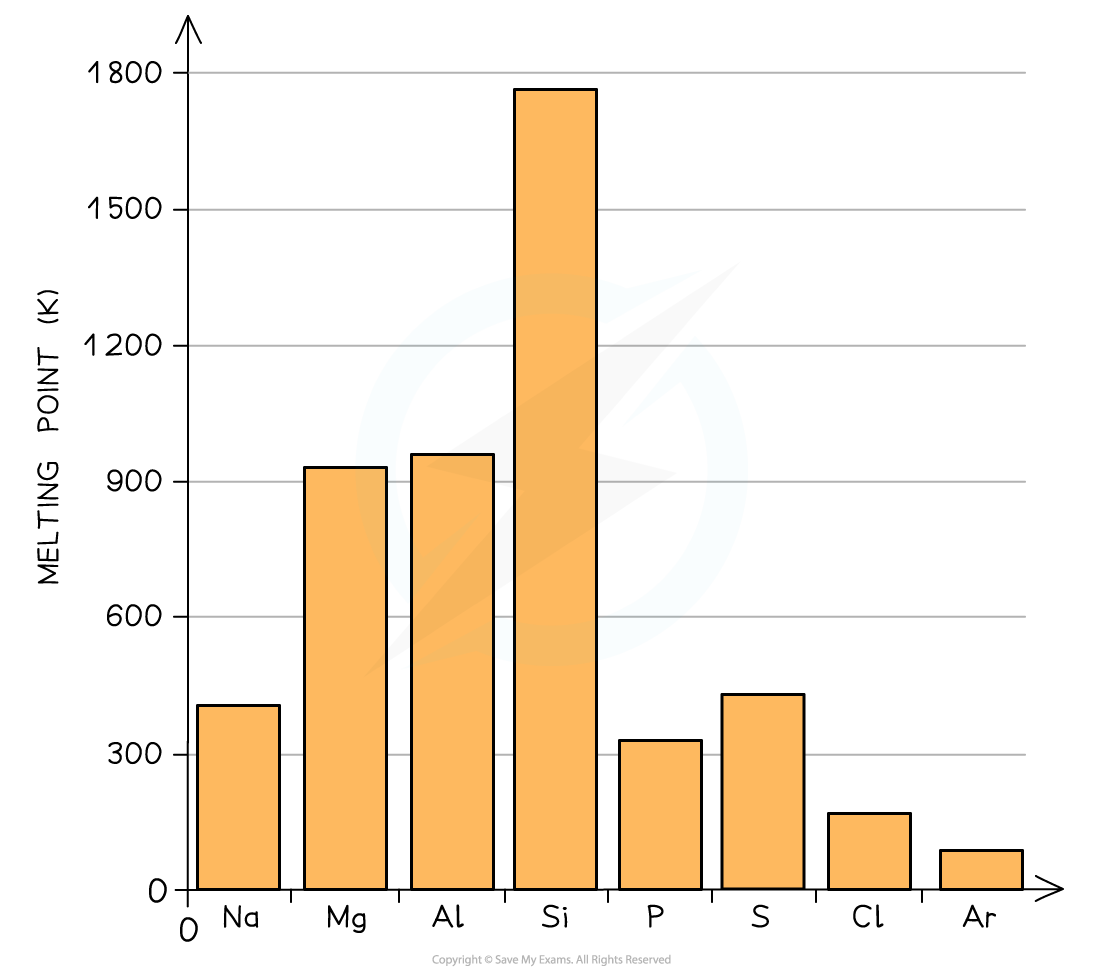
Melting points as you go across a period. The metallic bonding gets stronger from Na to Al
Melting points of metals down a group
- As you go down the group the size of the cation increases so this decreases the attraction between the valence electrons and the metallic lattice, leading to a reduction of the melting point
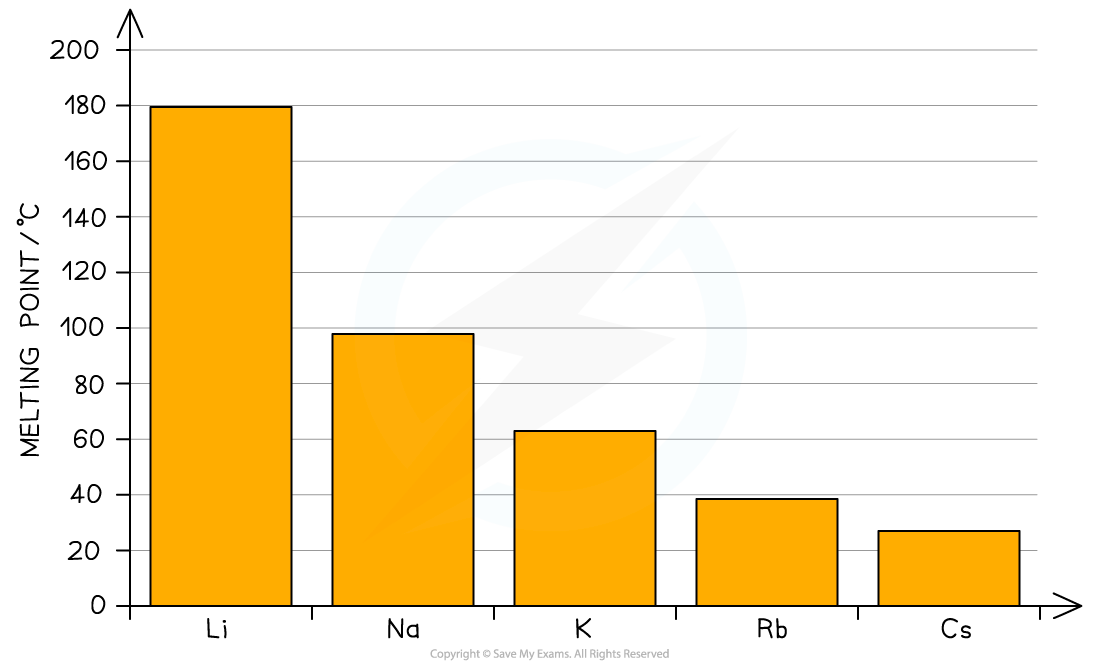 Melting points as you go down a group of metals. The metallic bonding gets weaker from Li to Cs
Melting points as you go down a group of metals. The metallic bonding gets weaker from Li to Cs
Exam Tip
You see from the graph that the melting pont of aluminium is not that much higher than magnesium. It is a reminder to us that these are trends and not rules about melting points and sometimes there are other factors which can result in subtle differences from what was expected.One factor here is the metal packing structure - this can also influence the melting point, but it is beyond what is required in the IB Chemistry syllabus. You just need to learn and explain the broad trends
转载自savemyexams


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








